2500 Safety in Chemistry Laboratory
- Page ID
- 440566
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)SAFETY AND THE CHEMICAL LABORATORY
1.0 INTRODUCTION
Teaching and research laboratories are locations for learning and exploring chemical reactions and properties. Like all activities in daily life, a risk of injury exists. Safety precautions and work practices reduce this risk. There are some hazards that are specific for chemicals.
This document cannot cover all laboratory safety guidelines for all future chemistry operations students will encounter. A whole field of industrial hygiene exists to create safer work practices. The objectives for this experiment are to
· Establish a culture of safety awareness
· Introduce the RAMP concept
· State the general rules for working safely in a chemical laboratory
· Describe differences between a hazard and a risk and acute and chronic toxicity
· Introduce a safety data sheet (SDS) and identify information it contains
· Present three common labeling and marking systems
· Build safe work practices into good habits
· Introduce regulatory agencies
· Prepare for emergency responses for when things happen
Chemical experiments in the teaching laboratory should contain a section on safety precautions and waste disposal. It is important to review these before entering the laboratory.
Safety precautions have been developed over many decades and are based on much experience and many events. The American Chemical Society (ACS) guidelines for undergraduate laboratory safety recommend the R. A. M. P. method.
· Recognize the hazards
· Assess the risks of hazards
· Minimize the risks of the hazards
· Prepare for emergencies from uncontrolled hazards
References and further reading
Guidelines of Chemical Laboratory Safety in Academic Institutions, ACS Committee on Chemical Safety (CCS) Task Force for Safety Education Guidelines, 2016.
National Fire Protection Association (NFPA), 2017 https://www.nfpa.org/Assets/files/Ab...4/704_FAQs.pdf (accessed 06/23/2020)
Emergency Response Guidebook (2016), U. S. Department of Transportation. https://www.phmsa.dot.gov/sites/phms...cs/ERG2016.pdf (accessed 06/23/2020)
Prudent Practices in the Laboratory, Handling and Management of Chemical Hazards, National Research Council Committee on Prudent Practices in the Laboratory, 2011
Chemical Hygiene Plan, Los Medanos College, (the current version in effect).
2.0 RECOGNIZE THE HAZARDS
2.1 CHEMICAL LABELING
To recognize the hazards of chemical, one of the most important things is to read the label, documents that are shipped with the chemical, and additional references. Several types of labeling systems are in use. Each has been developed over time and for specific reasons. Three labeling systems are described below, but there are a few more systems in specific industries:
A: National Fire Protection Association (NFPA)
The NFPA is an international non-profit organization that develops standards to reduce injury due to fire, electrical and related hazards. The NFPA developed a labeling system that is in common use from a fire service perspective. Federal, state or local regulations may require the use of the NFPA labeling system. It is common to see it on buildings such as water treatment facilities and medical facilities where elevated levels of oxygen are present.
The NFPA labeling consists of a diamond made of red, blue, yellow and white squares:

Figure 1: NFPA Diamond
Each color represents a specific and different hazard. Red is for flammability, blue is for health, yellow is for chemical reactivity and white is for special hazards. A number is shown from 0 (low) to 4 (high) in each of the squares that indicates a general assessment of the specific hazard. The NFPA diamond for acetone (formula C3H6O), a common organic solvent, is shown below:
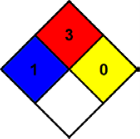
Figure 2: NFPA Diamond for Acetone
Acetone is moderately to highly flammable, and so is rated a '3' in the flammability diamond. Direct exposure can cause minor skin irritation, so it is rated a '1' in the health diamond. Acetone is not particularly chemically reactive, and so is rated as a '0' in the reactivity diamond. It has no special hazards, so the white diamond is left blank.
The NFPA labeling is designed for a quick and general overview of the safety concerns, especially for first responders. It is often displayed on buildings and storage facilities. Sometimes it can be seen on smaller containers.
B: U. S. Department of Transportation (DOT) Pipeline and Hazardous Materials Safety Administration (PHMSA)
When chemicals are transported along public highways, it is important for external packaging to display the hazards posed by the contents in the event of an accident and potential release. The PHMSA, a part of the DOT, regulates many aspects of this transportation, including but not limited to packaging, quantities and placarding. Some examples of the placards include the following:






Placards are often placed on trucks and railcars. These are often also displayed on shipping boxes for smaller quantities (in general for air transport) and for compressed gas cylinders.
Specific information for the transportation of chemicals can be found in the Emergency Response Guidebook (ERG). The ERG is updated every 4 years by the PHMSA. Most public safety agencies are required to have a hardcopy of the ERG or immediate access to the ERG in the event of an emergency.

Figure 3 ERG Covers
A link to the current ERG is included in the reference section. A mobile app is available.
If the placard contains a number, it is cross-referenced in the ERG with guides to additional information about the specific hazards for the classes of chemicals present. An example would be a placard showing corrosive with the number "1830". The "1830" is an identifier for concentrated sulfuric acid (in the yellow bordered pages of the ERG). It corresponds to Guide No. 137 in the orange pages which has information about "Substances - Water-Reactive Corrosive".
The ERG is a useful resource to cross-reference trade and industry names. It provides more specific information about classes of compounds than the NFPA diamond.
C: U. S. Department of Labor (DOL) Occupational Safety and Health Administration (OSHA) Globally Harmonized System (GHS)
The GHS is an international system of hazard identification. The GHS was required by OSHA to be fully implemented by 2016. GHS labels are not mutually exclusive with the NFPA and DOT systems. It is possible to see a GHS symbol, a DOT symbol and an NFPA diamond side by side. Because GHS is used internationally, the same set of symbols should be used on the same material anywhere on the globe.
There are 3 components to the GHS:
· Hazard Classification Statements
o Physical
o Health
o Environmental Hazard
· Labels and Standard Symbols
· Safety Data Sheets (SDS)
The chemical manufacturer or importer is responsible for determining the hazard classification for the material. There are physical, health, and environmental hazard statement general categories. Some materials may fall into multiple categories.
A listing of the 9 specific hazard classes and GHS pictograms are below:

The GHS standardized the format and contents of labels on chemicals from the manufacturer. Within the laboratory environment, the GHS hazard labels will be the system most commonly encountered.
The GHS pictograms provide a way to rapidly assess the general chemical hazard of material. Additional information is contained within documents called the Safety Data Sheets (SDSs). The GHS specifies a standard set of 16 sections and content for SDSs. The chemical manufacturer is responsible for producing the SDS that accurately describes the known hazards of the material within the GHS format. Employees working with hazardous material must have access to the SDS as part of several labor laws (both Federal and State).
Several online sources of SDS are available. Labor laws require that the documentation is available and accurately describes the hazard of the material. Although it is best to have the SDS from the manufacturer, if a SDS is missing or lost, a replacement SDS can be accessed from an alternative source. Most manufacturers provide SDS free of charge from their catalog website. Some possible sources include:
https://www.sigmaaldrich.com/united-states.html
https://www.fishersci.com/us/en/home.html
A full SDS for sulfuric acid is included in Appendix A. The SDS has 16 parts:
Table 1 Safety Data Sheet Sections
|
1. Name |
9. Physical and Chemical Properties |
|
2. Hazard(s) |
10. Stability and Reactivity |
|
3. Compositions |
11. Toxicological information |
|
4. First Aid |
12. Ecological Information |
|
5. Firefighting Measures |
13. Disposal Considerations |
|
6. Accidental Release Measures |
14. Transport information |
|
7. Handling and Storage |
15. Regulatory Information |
|
8. Exposure Controls/Personal Protection |
16. Other information |
One of the most important pieces of information provided in the SDS is the proper name and Chemical Abstracts Service (CAS) number for each pure component of the material. The CAS is a unique identifier for a compound and avoids confusion. Many industries use legacy names for chemicals, and these names are often used interchangeably. For example, isopropyl alcohol (also called 'rubbing alcohol') is the common name for 2-propanol. Even though these names are different, they all have the same CAS number of 67-63-0.
The Los Medanos College Chemistry Stockroom maintains a SDS for all pure compounds within the stockroom. A SDS will be provided upon request.
Reagents produced and distributed for use within the chemistry courses will have a GHS warning label, or the words 'No GHS Label Required', as appropriate.
Critical thinking skills are essential when reviewing a SDS. Unusual, rare or new compounds may have unknown hazards that are not included on the SDS. Manufacturers are conservative when describing hazards. Sometimes different manufacturers will assign different hazard classes to the same material. Consider the difference between sulfuric acid at different concentrations. Appendix B shows the SDS pages for different dilutions of sulfuric acid. While it would be expected that concentrated sulfuric acid will have the same GHS hazard class from all manufacturers, with diluted sulfuric acid there may be a judgment call made by the manufacturer.
2.2 ADDITIONAL HAZARDS
Like many work and school environments, laboratory hazards are not limited to chemical hazards. Wet floors are potential slip areas. Glassware can break, leaving sharp edges which can cut. Hot items often look like cold items and could result in accidental burns. Electrical hazards from equipment are commonly present.
Less common hazards include, but are not limited to, radioactive materials, cryogens (cold materials), high pressure systems, vacuum systems, energetic compounds (explosives), and air or water reactive materials. It is beyond the scope of this document to comprehensively describe safety procedures for less common hazards. Specific knowledge, safety procedures, equipment and careful consultation with experienced personnel is recommended before working with any of these hazards.
3.0 ASSESS THE RISKS OF HAZARDS
The amount of information included in safety documentation can be overwhelming. By establishing 9 hazard classes, the GHS system quickly allows a general assessment of the hazard of a material. Whenever an unfamiliar chemical is to be used in a new procedure, a thorough review of the SDS is recommended to accurately evaluate the risk of hazard.
Laboratory experiments within introductory chemistry courses have been tested and evaluated to minimize the risk to students, faculty and staff. Maintaining proper labeling and documentation is part of the training for all students and staff for assessing risks of materials.
Experiments sometimes have special warnings that are set apart from the main text. Personnel should be certain to read and understand these warnings. An example is shown below:
|
!!Special Warnings Will be Stated Here!! Experiments may include descriptions of specific hazards relevant to the experiment being planned. Be certain to read and understand these instructions. !!Disposal Instructions!! A description of how to dispose of the waste generated in the experiment should be included. Some waste may be permitted for drain disposal. Other waste must be captured for off-site disposal. Waste types are often segregated to prevent side reactions. Some waste is acutely hazardous and is easier and cheaper to manage in smaller quantities. Refer to this section in the specific experiment. Consider the generation, management and disposal of waste generated by laboratory processes. Sometimes, the disposal costs for chemical reagents can be several times the purchase price of the original quantity. |
4.0 MINIMIZE THE RISKS OF THE HAZARDS
Work practices can be designed to minimize the risks of many types of hazards within the chemistry laboratory. These measures include but are not limited to:
· Creation of experimental procedures and standard operating procedures
· Evaluation of experimental design
· Substitution of less hazardous materials when feasible
· Engineering controls such as fume hoods, separate laboratory classrooms
· Personal Protective Equipment (PPE) appropriate to the operation
By building good work habits within the chemistry laboratory course, risks are minimized. Some good habits include:
· Read and learn about the experiment before the course session
· Wear protective eyewear
· Wear protective clothing
o Closed-toe shoes
o Long pants
o Lab coat
· Tie loose clothing and long hair
· Use specialized waste containers as appropriate
o Broken glass waste containers
o Biohazard disposal bags
o Sharps disposal containers (for syringe needles)
· Segregate waste for disposal
· Keep areas clear from clutter
· Never leave open flames unattended
· Use fume hoods if appropriate
The creation, review, and incorporation of this Safety in the Chemistry Laboratory procedure and familiarization by personnel is part of the minimization of risk.
5.0 PREPARE FOR EMERGENCIES FROM UNCONTROLLED HAZARDS
While the previously described documentation, labels, procedures and good work practices are very effective at reducing emergencies, failures and mishaps will occur. Glass equipment may experience hairline fractures resulting in breaks. Spills may occur at any time. Electrical equipment may provide sparks and ignition sources for flammable materials.
All personnel should be prepared for emergencies and should be familiar with safety equipment including:
· Fire extinguishers
· Eyewash stations
· Safety showers
· Spill kits
· First aid kits
· Fire alarms
· Evacuation routes
· Emergency telephone numbers
Equipment should be inspected on a regular basis. Inspection tags and other documentation should be retained for a time to indicate that equipment has been inspected and is operational. At some facilities, emergency drills are used to test and exercise the emergency response system.
6.0 ACCIDENT INVESTIGATION
When an accident does occur, a report should be made to the instructor or supervisor. A review may be initiated. The review may be simple or complex and depends upon the severity of the accident. For example, a dropped beaker that breaks and does not injure anyone may need a simple cleanup, disposal and replacement. However, a chemical splash into a person's eye will require at least 15 minutes of flushing with water followed by a medical evaluation and a more comprehensive investigation.
The accident investigation process should be viewed to determine a root cause of the underlying accident. In some cases, the root cause may not be identified, or it cannot be addressed (such as with types of equipment failures). It is important for all people involved in an accident investigation to understand that the purpose is not to establish blame or responsibility, but the root cause (if any) so conditions that led to the accident can be changed to avoid future recurrences.
7.0 Activities
1. Choose one experiment in this laboratory manual that uses chemical reagents. Find a Safety Data Sheet for each reagent in the experiment. List the reagent, CAS number and GHS Warning Symbol for all reagents below:
|
Experiment Number |
|||
|
Chemical |
CAS |
GHS Warning Symbol |
Notes |
2. Select one of the above reagents. Find a second SDS from an alternative supplier. Are they any significant differences between the two SDS documents? Would you expect any differences? Why or why not?
3. On the SDS provided in Appendix A, select an abbreviation or acronym. Find the full definition and share with your class what it is, what it means, and how should it be interpreted (e.g, LD50, IDLH, TWA, ACGIH, NIOSH, CFR, OSHA, etc.).
4. Draw a map of the laboratory classroom. Include the following:
· Where your lab station is located
· Exit/entrance doors
· Sinks
· Fume Hoods
· Eyewash station
· Safety Showers
· Fire Extinguishers
· Fire Alarm locations
· Emergency notification equipment
Appendix A: Safety Data Sheet for Concentrated Sulfuric Acid
Appendix B: First Pages of Additional SDS for Sulfuric Acid (various dilutions)

