Learning Objective
- Learn the basic terms used to describe matter
The definition of chemistry—the study of the interactions of matter with other matter and with energy—uses some terms that should also be defined. We start the study of chemistry by defining some basic terms.
Matter
Matter is anything that has mass and takes up space. A book is matter, a computer is matter, food is matter, and dirt in the ground is matter. Sometimes matter may be difficult to identify. For example, air is matter, but because it is so thin compared to other matter (e.g., a book, a computer, food, and dirt), we sometimes forget that air has mass and takes up space. Things that are not matter include thoughts, ideas, emotions, and hopes.
Example \(\PageIndex{1}\)
Which of the following is matter and not matter?
- a hot dog
- love
- a tree
Solution
- A hot dog has mass and takes up space, so it is matter.
- Love is an emotion, and emotions are not matter.
- A tree has mass and takes up space, so it is matter.
Exercise \(\PageIndex{1}\)
Which of the following is matter and not matter?
- the moon
- an idea for a new invention
Answer
- The moon is matter.
- The invention itself may be matter, but the idea for it is not.
To understand matter and how it changes, we need to be able to describe matter. There are two basic ways to describe matter: physical properties and chemical properties.
Physical properties
Physical properties are characteristics that describe matter as it exists. Some of the many physical characteristics of matter are shape, color, size, and temperature. An important physical property is the phase (or state) of matter. The three fundamental phases of matter are solid, liquid, and gas (Figure \(\PageIndex{1}\)).

Figure \(\PageIndex{1}\): The Phases of Matter. Chemistry recognizes three fundamental phases of matter: solid (left), liquid (middle), and gas (right). Image used with permission (CC BY-SA 3.0; Spirit469)
Chemical Properties
A characteristic that describes how matter changes form in the presence of other matter is a chemical property. Does a sample of matter burn? Burning is a chemical property, the matter will only burn in the presence of oxygen so requires the presence of some other type of matter to undergo this chemical change. Does it behave violently when put in water? Reactivity with water is a chemical property as well (Figure \(\PageIndex{2}\)). In the following chapters, we will see how descriptions of physical and chemical properties are important aspects of chemistry.

Figure \(\PageIndex{2}\): Chemical Properties. The fact that this match burns is a chemical property of the match. Image used with permission (Sebastian Ritter (Rise0011))
Physical Change
If matter always stayed the same, chemistry would be rather boring. Fortunately, a major part of chemistry involves change. A physical change occurs when a sample of matter changes one or more of its physical properties. For example, a solid may melt (Figure \(\PageIndex{3}\)), or alcohol in a thermometer may change volume as the temperature changes. A physical change does not affect the chemical composition of matter.

Figure \(\PageIndex{2}\): Physical Changes: The solid ice melts into liquid water—a physical change. A time-lapse animation of icecubes melting in a glass (50 minutes). Image used with permission (Public Domain; Moussa).
Chemical change
A chemical change is the process of demonstrating a chemical property, such as the burning match in Figure 1.2.2 "Chemical Properties". As the matter in the match burns, its chemical composition changes, and new forms of matter with new physical properties are created. Note that chemical changes are frequently accompanied by physical changes, as the new matter will likely have different physical properties from the original matter.
Example \(\PageIndex{2}\)
Describe each process as a physical change or a chemical change.
- Water in the air turns into snow.
- A person’s hair is cut.
- Bread dough becomes fresh bread in an oven.
Solution
- Because the water is going from a gas phase to a solid phase, this is a physical change.
- Your long hair is being shortened. This is a physical change.
- Because of the oven’s temperature, chemical changes are occurring in the bread dough to make fresh bread. These are chemical changes. (In fact, a lot of cooking involves chemical changes.)
Exercise \(\PageIndex{2}\)
Identify each process as a physical change or a chemical change.
- A fire is raging in a fireplace.
- Water is warmed to make a cup of coffee.
Answers
- chemical change
- physical change
Substance
A sample of matter that has the same physical and chemical properties throughout is called a substance. Sometimes the phrase pure substance is used, but the word pure isn’t needed. The definition of the term substance is an example of how chemistry has a specific definition for a word that is used in everyday language with a different, vaguer definition. Here, we will use the term substance with its strict chemical definition.
Chemistry recognizes two different types of substances: elements and compounds.
Element
An element is the simplest type of chemical substance; it cannot be broken down into simpler chemical substances by ordinary chemical means. There are about 115 elements known to science, of which 80 are stable. (The other elements are radioactive, a condition we will consider in Chapter 15.) Each element has its own unique set of physical and chemical properties. Examples of elements include iron, carbon, and gold.
Compound
A compound is a combination of more than one element. The physical and chemical properties of a compound are different from the physical and chemical properties of its constituent elements; that is, it behaves as a completely different substance. For example water, H2O, is made up of oxygen and hydrogen. Both oxygen and hydrogen are extremely flammable elements but once we allow them to react with each other we get nonflammable water. There are over 50 million compounds known, and more are being discovered daily. Examples of compounds include water, sugar, penicillin, and sodium chloride (the chemical name for common table salt).
Mixtures
Elements and compounds are not the only ways in which matter can be present. We frequently encounter objects that are physical combinations of more than one element or compound. Physical combinations of more than one substance are called mixtures. There are two types of mixtures.
Heterogeneous mixture
A mixture composed of two or more substances. is a mixture composed of two or more substances. It is easy to tell, sometimes by the naked eye, that more than one substance is present.
Homogeneous mixture/ Solution
A homogeneous mixture is combination of two or more substances that are so intimately mixed that the mixture behaves as a single substance. Another word for a homogeneous mixture is solution. Thus, a combination of salt and steel wool is a heterogeneous mixture because it is easy to see which particles of the matter are salt crystals and which are steel wool. On the other hand, if you take salt crystals and dissolve them in water, it is very difficult to tell that you have more than one substance present just by looking—even if you use a powerful microscope. The salt dissolved in water is a homogeneous mixture, or a solution (Figure \(\PageIndex{3}\)).

Figure \(\PageIndex{3}\): Types of Mixtures © Thinkstock On the left, the combination of two substances is a heterogeneous mixture because the particles of the two components look different. On the right, the salt crystals have dissolved in the water so finely that you cannot tell that salt is present. The homogeneous mixture appears like a single substance.
Example \(\PageIndex{3}\)
Identify the following combinations as heterogeneous mixtures or homogenous mixtures.
- soda water (Carbon dioxide is dissolved in water.)
- a mixture of iron metal filings and sulfur powder (Both iron and sulfur are elements.)

Figure \(\PageIndex{4}\): A mixture of iron filings and sulfur powder (Asoult, Fe-S mixture 03, CC BY 4.0)
Solution
- Because carbon dioxide is dissolved in water, we can infer from the behavior of salt crystals dissolved in water that carbon dioxide dissolved in water is (also) a homogeneous mixture.
- Assuming that the iron and sulfur are simply mixed together, it should be easy to see what is iron and what is sulfur, so this is a heterogeneous mixture.
Exercise \(\PageIndex{3}\)
Are the following combinations homogeneous mixtures or heterogeneous mixtures?
- the human body
- an amalgam, a combination of some other metals dissolved in a small amount of mercury
Answers
- heterogeneous mixture
- homogeneous mixture
There are other descriptors that we can use to describe matter, especially elements. We can usually divide elements into metals and nonmetals, and each set shares certain (but not always all) properties.
Metals are elements that are solid at room temperature (although mercury is a well-known exception), are shiny and silvery, conducts electricity and heat well, can be pounded into thin sheets (a property called malleability), and can be drawn into thin wires (a property called ductility).
Nonmetals are elements which are brittle when solid, do not conduct electricity or heat very well, and cannot be made into thin sheets or wires (Figure \(\PageIndex{5}\)). Nonmetals also exist in a variety of phases and colors at room temperature.
Some elements have properties of both metals and nonmetals and are called semimetals (or metalloids). We will see later how these descriptions can be assigned rather easily to various elements.

Figure \(\PageIndex{5}\): Semimetals © Thinkstock On the left is some elemental mercury, the only metal that exists as a liquid at room temperature. It has all the other expected properties of a metal. On the right, elemental sulfur is a yellow nonmetal that usually is found as a powder.
Describing Matter Flowchart
"Describing Matter" is a flowchart of the relationships among the different ways of describing matter.
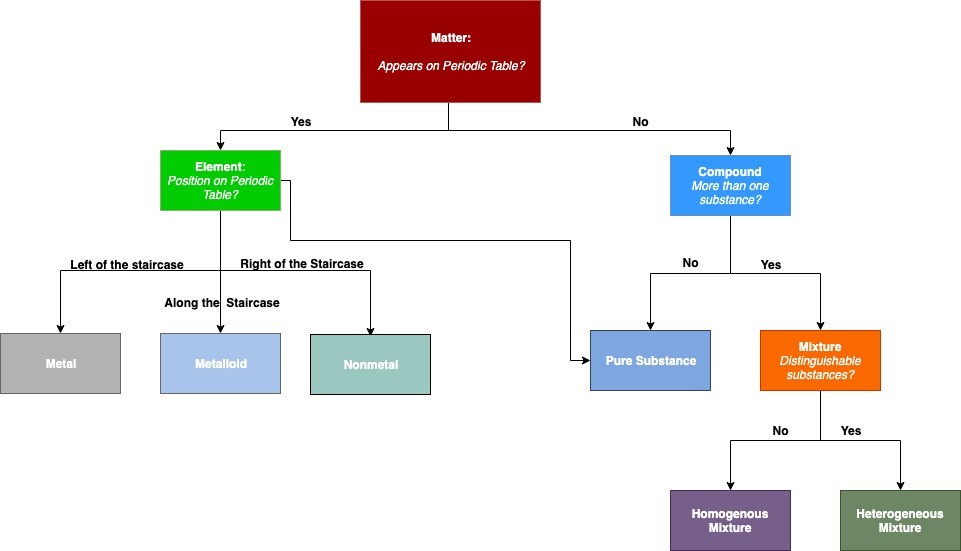
Figure \(\PageIndex{6}\): Describing Matter. This flowchart shows how matter can be described.
Example \(\PageIndex{1}\): Chemistry Is Everywhere: In the Morning
Most people have a morning ritual, a process that they go through every morning to get ready for the day. Chemistry appears in many of these activities.
- If you take a shower or bath in the morning, you probably use soap, shampoo, or both. These items contain chemicals that interact with the oil and dirt on your body and hair to remove them and wash them away. Many of these products also contain chemicals that make you smell good; they are called fragrances.
- When you brush your teeth in the morning, you usually use toothpaste, a form of soap, to clean your teeth. Toothpastes typically contain tiny, hard particles called abrasives that physically scrub your teeth. Many toothpastes also contain fluoride, a substance that chemically interacts with the surface of the teeth to help prevent cavities.
- Perhaps you take vitamins, supplements, or medicines every morning. Vitamins and other supplements contain chemicals your body needs in small amounts to function properly. Medicines are chemicals that help combat diseases and promote health.
- Perhaps you make some fried eggs for breakfast. Frying eggs involves heating them enough so that a chemical reaction occurs to cook the eggs.
- After you eat, the food in your stomach is chemically reacted so that the body (mostly the intestines) can absorb food, water, and other nutrients.
- If you drive or take the bus to school or work, you are using a vehicle that probably burns gasoline, a material that burns fairly easily and provides energy to power the vehicle. Recall that burning is a chemical change.
These are just a few examples of how chemistry impacts your everyday life. And we haven’t even made it to lunch yet!

Figure \(\PageIndex{6}\): Chemistry in Real Life © Thinkstock. Examples of chemistry can be found everywhere—such as in personal hygiene products, food, and motor vehicles.
Key Takeaways
- Chemistry is the study of matter and its interactions with other matter and energy.
- Matter is anything that has mass and takes up space.
- Matter can be described in terms of physical properties and chemical properties.
- Physical properties and chemical properties of matter can change.
- Matter is composed of elements and compounds.
- Combinations of different substances are called mixtures.
- Elements can be described as metals, nonmetals, and semimetals.









