10.3: Organic Structural Representations: Expanded Structural Notation
- Page ID
- 237748
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Define expanded structural notation.
- Define constitutional isomer.
- Represent an organic molecule using expanded structural notation.
- Identify the compositional characteristics of a molecule that can be determined by analyzing a structure that is represented using expanded structural notation.
As stated previously, organic chemists use several different notations, including molecular formulas and chemical names, to represent organic molecules. While a molecular formula indicates both the types and quantities of elements that are present in a molecule, this organic structural representation does not provide any information about the connectivity, or relative bonding arrangement, of those elements. Furthermore, neither the number, type, or location of the bonds that are present in the corresponding structure, nor the quantity or location of lone pairs that are found in the molecule, can be determined by analyzing a molecular formula. Therefore, in order to obtain more information about the compositional characteristics of an organic chemical, the structure of that substance must be represented in a visual format.
Since, by definition, organic substances are covalently-bonded molecules, their structures can be visually-represented using expanded structural notation, which is a modified version of a two-dimensional Lewis structure. Recall that the presence of unpaired electrons within an atom is inherently destabilizing. Therefore, if two atoms that contain unpaired electrons approach one another, those electrons will interact with one another to form a shared pair of electrons, which are represented as lines in a Lewis structure. Any paired electrons that are present in an atom are already sufficiently stable and, consequently, do not form bonds. Instead, those electrons will remain as lone, or unshared, pairs, which are represented as dots in Lewis structures.
In order to determine the number of bonds and lone pairs that will be associated with a given atom when that element is incorporated into an organic molecule, the electron dot structure of that element must be drawn. Electron dot structures surround the elemental symbol of an element with one dot for every valence electron that the element contains. When drawing an electron dot structure, the first dot can be placed on any "side" of the elemental symbol (top, bottom, left, or right), and the first four dots must each be placed on their own "side" of the elemental symbol. In other words, if the first dot is placed on the top of the elemental symbol, the second dot can be placed on the bottom, left, or right of the symbol, but cannot be placed at the top, alongside the first dot. The remaining dots can again be placed on any "side" of the elemental symbol, but must be arranged such that no more than two dots exist on any "side" of the elemental symbol. Figure \(\PageIndex{1}\) shows a chemically-correct electron dot structure for each of the non-metals that can be incorporated into an organic molecule.

As stated above, one covalent bond will form for every unpaired valence electron that is present in a given atom. Therefore, carbon, C, which contains four unpaired valence electrons, can form four covalent bonds. Because hydrogen, H, only bears one unpaired valence electron, this element will be associated with one bond when incorporated into a covalent molecule. Recall that hydrogen is unable to achieve an octet configuration when bonding, due to the inherent deficiency in its atomic electron configuration. Therefore, this element, which achieves a duet configuration when bonding, is classified as "electron deficient." Nitrogen, N, and phosphorus, P, each contain three unpaired valence electrons and, therefore, can form three covalent bonds. Furthermore, the electron dot structures of nitrogen and phosphorus also contain one set of paired electrons. Therefore, when these elements are incorporated into a molecule that is represented using expanded structural notation, one lone pair must be drawn on their corresponding elemental symbols. Oxygen, O, and sulfur, S, will each be associated with two bonds and two lone pairs when incorporated into a covalent substance, and the halogens, X, will each bear one bond and three lone pairs when present in an organic molecule.
Because all organic molecules must contain carbon, C, this element should be written first when symbolizing an organic substance using expanded structural notation. In order to represent the bond that exists between consecutive atoms, a line that connects their corresponding elemental symbols should be drawn. As stated above, when drawing an electron dot structure, equivalent images can be generated by placing the first dot on any "side" of the elemental symbol (top, bottom, left, or right), and the next three dots must each be placed on their own "side" of the elemental symbol. Similarly, when connecting atoms using expanded structural notation, the second carbon can be bonded to any "side" of the first elemental symbol that is written, but the connectivity of all subsequent carbon atoms must be considered relative to those that are already present. Additional lines should then be drawn on each elemental symbol, until a total of four bonds, which, as indicated above, corresponds to the preferred bonding configuration of carbon, are represented. Any heteroatoms that are contained in the molecule that is being symbolized should then be incorporated by writing their elemental symbols on the lines that are present in the structure that is being generated. Additional lines should then be drawn on each of those elemental symbols, as necessary, until the appropriate number of bonds for that element are represented. The elemental symbol for hydrogen, H, should then be written in all of the unoccupied positions at the ends of the bonds that have been drawn, and, finally, the correct number of lone pairs should be added to any heteroatom symbols.
For example, represent an organic alkane that contains four hydrogens, two carbons, and two chlorines using expanded structural notation.
Because all organic molecules must contain carbon, C, the two carbon atoms that are present in this molecule are written first. Since the given molecule is described as an alkane, only single bonds can be used to connect the atoms in the structure that is being developed. Because, as stated above, the second carbon can be bonded to any "side" of the first elemental symbol that is written, each of the three images that are shown below are chemically-equivalent to one another.
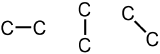
Each of the carbons that are shown in these images are currently associated with one bond. Therefore, because carbon forms four bonds when present in an organic molecule, three additional lines must be drawn on each "C" in the structure that is being developed. The following image results upon modifying the first structure that is shown above to reflect these additional bonds.
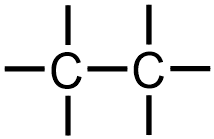
Because the given molecule contains chlorine, which is classified as a halogen and, therefore, as a heteroatom, two "Cl" symbols should be incorporated into this structure. While the first chlorine can be written on any of the six lines that are present in the structure that is shown above,
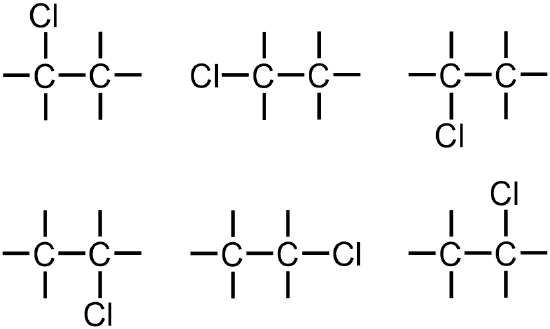
the connectivity of the second chlorine atom must be considered relative to both the carbons and the chlorine that are already present. A total of 15 structures can be drawn from the images that are shown above. However, the six structures that are shown in red and purple are chemically-identical to one another, because both of the chlorines are bonded to the same carbon in each of these images. While the first three structures may seem unique, relative to the next three pictures, "flipping" one of the first three pictures by 180 degrees yields the picture that is shown directly below the initially-chosen structure. Similarly, the remaining nine structures, which are shown in green, also represent the same organic molecule, because each of the chlorines are bonded to different carbons. However, these two sets of images are distinct, relative to each other, due to the placement of the chlorine atoms on the same carbon or on different carbons, respectively. Therefore, the two structures that are boxed in blue are constitutional isomers, which are defined as organic molecules that have the same molecular formula but different relative connectivities. Each of the chlorines in the boxed molecules are associated with one bond, which is the preferred bonding configuration of a halogen. Therefore, no additional lines must be drawn on their corresponding elemental symbols.
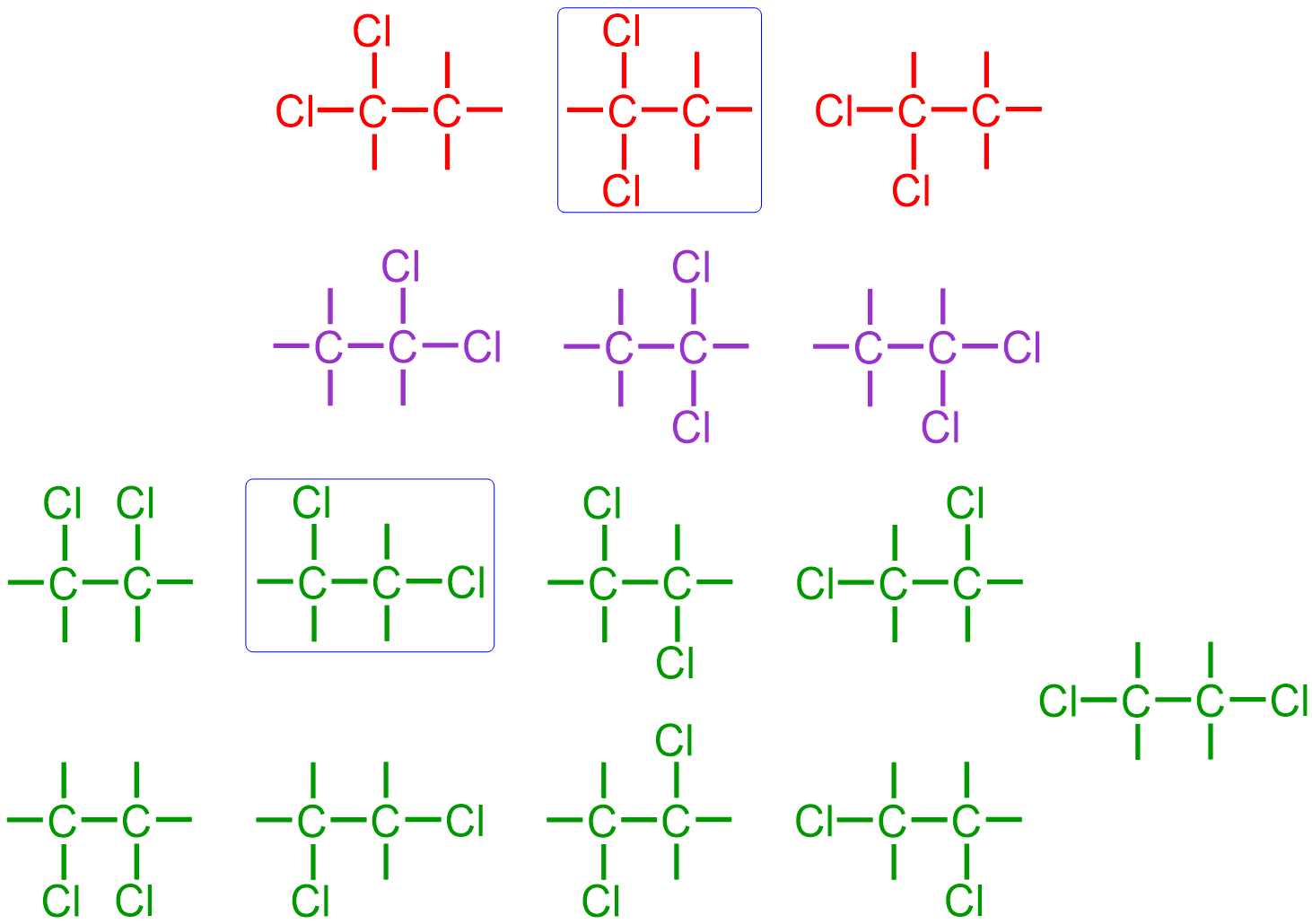
Finally, the boxed structures are completed by writing the elemental symbol for hydrogen, H, in all of the unoccupied positions at the ends of the bonds that have been drawn and adding three lone pairs to the elemental symbol of each chlorine. The following structures are both chemically-correct expanded structural representations of an organic alkane that contains four hydrogens, two carbons, and two chlorines.
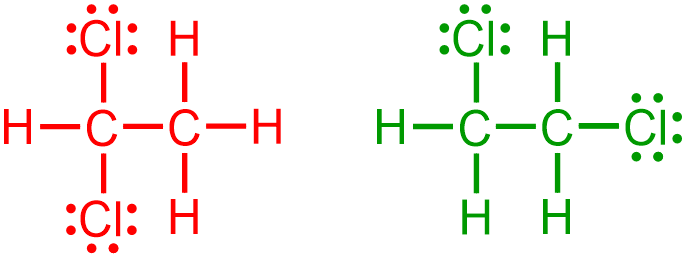
Nearly all of the compositional characteristics of a molecule can be determined by analyzing a structure that is symbolized using expanded structural notation. The types of elements that are present are indicated by the elemental symbols that are shown in the corresponding structural representation. The quantity of each element, as well as the total number of bonds and lone pairs that are present in a molecule, can be established by counting the number of times that each symbol is written in a molecular image. Additionally, the connectivity, or relative bonding arrangement, of each atom, bond, and lone pair can be explained by describing the placement of each of these components, relative to one another, in an organic structural representation. Finally, the types of bonds that are found in a molecule can be determined by counting the number of lines that are drawn between any two atoms in the corresponding image.

