3.21: Covalent Bonding: Multiple Bonds
- Page ID
- 213223
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain how double and triple bonds are formed in covalent molecules.
- Draw Lewis structures of covalent molecules that contain double and triple bonds.
- Identify the types of bonds that are present within a covalent molecule.
The process for drawing the Lewis structures of covalent molecules has been presented and applied in multiple sections within this chapter. In every example that was presented, the electron dot structures for a given combination of elements were drawn, and the number of unpaired electrons contained within each was determined, in order to assign the relative placement of these atoms within the final covalent molecule. Then, covalent bonds, or shared pairs of electrons, were generated by pairing these unpaired electrons. In all cases, creating a single shared pair of electrons between each set of atoms both paired all of the unpaired electrons that were present in the initially-drawn electron dot structures and satisfied the valences of all of the atoms involved in the pairing process. The resultant structures were only stable and, therefore, chemically-correct if both of these requirements were met. Two chemically-correct structures of sulfur difluoride, which were first generated in Section 3.15, are reproduced below. The left-most structure represents both shared pairs of electrons as dots, which are redrawn as lines, or bonds, in the right-most structure. Sulfur difluoride contains two single bonds, as only one shared pair of electrons exists between the central sulfur atom and each surrounding fluorine atom.

However, in some cases, creating only single bonds will not generate structures that meet the above-mentioned stability criteria. As a result, additional electron pairings must occur between the same set of atoms. A double bond is produced if two shared pairs of electrons exist between the same pair of atoms, and a triple bond is created by pairing three sets of electrons between the same atoms. The process for generating multiple bonds will be described in the following paragraphs.
Double Bonds
For example, consider the homonuclear diatomic covalent molecule named molecular oxygen, whose chemical formula is O2.
Recall that for a covalent molecule, the information represented in its chemical formula must be a direct reflection of its Lewis structure. Therefore, based on the elemental symbol and subscript shown in the given chemical formula, the Lewis structure for this molecule must contain only two oxygen atoms. Since oxygen is found in Group 6A of the periodic table, it contains 6 valence electrons. Two chemically-correct electron dot structures for this element are shown below.

Based on the structures shown above, each oxygen atom has 2 unpaired electrons. Typically, the element with more unpaired electrons becomes the central atom in a Lewis structure, and the other element is used as the surrounding atom. However, in the current example, each oxygen atom has the same number of unpaired electrons. As a result, neither can be designated as the central atom. Instead, a shared pair of electrons is created by pairing one unpaired electron from each oxygen atom. When executing this pairing step, the electron dot structure for one of the atoms should be rotated so that one of its unpaired electrons aligns with an unpaired electron on the other atom. Because the dots on an electron dot structure can be placed on any "side" of the elemental symbol, this rotation changes the orientation of the structure, but does not alter its meaning. A structure that results upon correctly executing this pairing process is shown below.

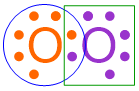
This structure contains one shared pair of electrons, which was created by pairing one unpaired electron from each oxygen atom. As this electron pair is located in between both oxygen electron dot structures, these electrons contribute to the overall electron configuration of both atoms. The remaining electrons only impact the electron configuration of the atom on which they are drawn. Therefore, each oxygen atom in the structure shown above is only surrounded by a total of seven dots. This information is visually-highlighted in the structure shown below using a blue circle around one oxygen atom and a green box around the other oxygen atom.
Since oxygen is found in the second period of the periodic table, it must adhere to the octet rule, without exception. Furthermore, one unpaired electron still exists on each oxygen atom. Because this structure contains atoms with unpaired electrons and unsatisfied valences, its electron arrangement is neither stable nor chemically-correct. These electronic deficiencies cannot be remedied by incorporating additional oxygen atoms, because the information represented in the given chemical formula, O2, must be a direct reflection of the Lewis structure that is ultimately generated. Therefore, in order to satisfy the valences of each of these atoms, the remaining unpaired electrons on each oxygen atom must be paired, in order to create a second shared pair of electrons. When executing this second pairing step, the electron dot structures cannot be rotated, due to the shared pair of electrons that has already been created. Therefore, the second shared pair of electrons is simply relocated to the space in between both oxygen electron dot structures. A structure that results upon correctly executing a second electron pairing process is shown below.

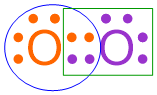
This structure contains two shared pairs of electrons, which were created by pairing two unpaired electrons from each oxygen atom. As these electron pairs are in between both oxygen electron dot structures, all four electrons contribute to the overall electron configuration of both atoms. The remaining electrons only impact the electron configuration of the atom on which they are drawn. By correctly executing a second pairing process, each oxygen atom is now surrounded by a total of eight, fully-paired dots. This information is visually-highlighted in the structure shown below using a blue oval around one oxygen atom and a green box around the other oxygen atom. As an octet configuration is the most stable electron arrangement that can be achieved by an atom, this structure represents the most stable bonding arrangement that can be achieved by combining two oxygen atoms.
Finally, in order to generate a structure that is more visually-appealing, each shared pair of electrons is replaced with a line that connects the adjacent elemental symbols. Because the structure above contains two pairs of electrons shared between the same pair of atoms, two lines must be drawn between these atoms in the corresponding Lewis structure. The remaining electrons are redrawn as dots, as shown below.
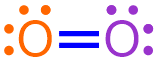
The structure shown above, which is a chemically-correct representation of a covalent compound, is a Lewis structure that represents the molecule that is formed when two oxygen atoms bond with one another. The remaining pairs of dots are called lone pairs, and the lines represent covalent bonds, or shared pairs of electrons. Because both lines are located between the same pair of atoms, this molecule contains a double bond.
Triple Bonds
The electron pairing process that was employed above to explain the formation of a double bond can be expanded upon to rationalize how triple bonds are created, as illustrated in Example \(\PageIndex{1}\) below.
Explain why the homonuclear diatomic covalent molecule named molecular nitrogen, whose chemical formula is N2, contains a triple bond.
Solution
Based on the elemental symbol and subscript shown in the given chemical formula, the Lewis structure for this molecule must contain only two nitrogen atoms. Since nitrogen is found in Group 5A of the periodic table, it contains 5 valence electrons. Two chemically-correct electron dot structures for this element are shown below.

Based on the structures shown above, each nitrogen atom has 3 unpaired electrons. Typically, the element with more unpaired electrons becomes the central atom in a Lewis structure, and the other element is used as the surrounding atom. However, in the current example, each nitrogen atom has the same number of unpaired electrons. As a result, neither can be designated as the central atom. Instead, a shared pair of electrons is created by pairing one unpaired electron from each nitrogen atom. When executing this pairing step, the electron dot structure for one of the atoms should be rotated so that one of its unpaired electrons aligns with an unpaired electron on the other atom. Because the dots on an electron dot structure can be placed on any "side" of the elemental symbol, this rotation changes the orientation of the structure, but does not alter its meaning. A structure that results upon correctly executing this pairing process is shown below.

This structure contains one shared pair of electrons, which was created by pairing one unpaired electron from each nitrogen atom. As this electron pair is located in between both nitrogen electron dot structures, these electrons contribute to the overall electron configuration of both atoms. The remaining electrons only impact the electron configuration of the atom on which they are drawn. Therefore, each nitrogen atom in the structure shown above is only surrounded by a total of six dots. This information is visually-highlighted in the structure shown below using a blue circle around one nitrogen atom and a green box around the other nitrogen atom.

Since nitrogen is found in the second period of the periodic table, it must adhere to the octet rule, without exception. Furthermore, two unpaired electrons still exist on each nitrogen atom. Because this structure contains atoms with unpaired electrons and unsatisfied valences, its electron arrangement is neither stable nor chemically-correct. These electronic deficiencies cannot be remedied by incorporating additional nitrogen atoms, because the information represented in the given chemical formula, N2, must be a direct reflection of the Lewis structure that is ultimately generated. Instead, a second shared pair of electrons is created by pairing an additional unpaired electron from each nitrogen atom. When executing this second pairing step, the electron dot structures cannot be rotated, due to the shared pair of electrons that has already been created. Therefore, the second shared pair of electrons is simply relocated to the space in between both nitrogen electron dot structures. A structure that results upon correctly executing a second electron pairing process is shown below.

This structure contains two shared pairs of electrons, which were created by pairing two unpaired electrons from each nitrogen atom. As these electron pairs are in between both nitrogen electron dot structures, all four electrons contribute to the overall electron configuration of both atoms. The remaining electrons only impact the electron configuration of the atom on which they are drawn. Therefore, each nitrogen atom in the structure shown above is only surrounded by a total of seven dots. This information is visually-highlighted in the structure shown below using a blue oval around one nitrogen atom and a green box around the other nitrogen atom.

As stated above, since nitrogen is found in the second period of the periodic table, it must adhere to the octet rule, without exception. Furthermore, one unpaired electron still exists on each nitrogen atom. Because this structure contains atoms with unpaired electrons and unsatisfied valences, its electron arrangement is neither stable nor chemically-correct. These electronic deficiencies still cannot be remedied by incorporating additional nitrogen atoms, because the information represented in the given chemical formula, N2, must be a direct reflection of the Lewis structure that is ultimately generated. Therefore, in order to satisfy the valences of each of these atoms, the remaining unpaired electrons on each nitrogen atom must be paired, in order to create a third shared pair of electrons. When executing this third pairing step, the electron dot structures cannot be rotated, due to the shared pairs of electrons that have already been created. Therefore, the third shared pair of electrons is simply relocated to the space in between both nitrogen electron dot structures. A structure that results upon correctly executing a third electron pairing process is shown below.

This structure contains three shared pairs of electrons, which were created by pairing three unpaired electrons from each nitrogen atom. As these electron pairs are in between both nitrogen electron dot structures, all six electrons contribute to the overall electron configuration of both atoms. The remaining electrons only impact the electron configuration of the atom on which they are drawn. By correctly executing a third pairing process, each nitrogen atom is now surrounded by a total of eight, fully-paired dots. This information is visually-highlighted in the structure shown below using a blue oval around one nitrogen atom and a green box around the other nitrogen atom. As an octet configuration is the most stable electron arrangement that can be achieved by an atom, this structure represents the most stable bonding arrangement that can be achieved by combining two nitrogen atoms.

Finally, in order to generate a structure that is more visually-appealing, each shared pair of electrons is replaced with a line that connects the adjacent elemental symbols. Because the structure above contains three pairs of electrons shared between the same pair of atoms, three lines must be drawn between these atoms in the corresponding Lewis structure. The remaining electrons are redrawn as dots, as shown below.

The structure shown above, which is a chemically-correct representation of a covalent compound, is a Lewis structure that represents the molecule that is formed when two nitrogen atoms bond with one another. The remaining pairs of dots are called lone pairs, and the lines represent covalent bonds, or shared pairs of electrons. Because all three lines are located between the same pair of atoms, this molecule contains a triple bond.
Consider each of the following Lewis structures.
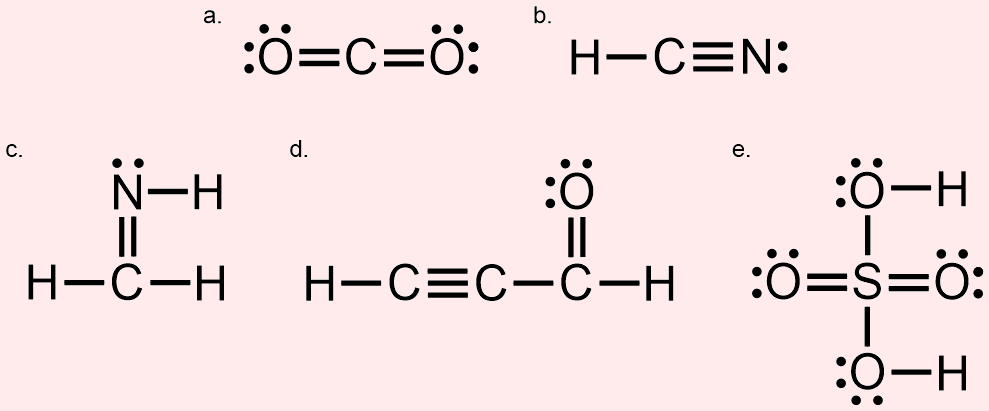
For each, state the ___________________ that are present within the corresponding molecule.
- total number of bonds
- type(s) of bonds
- number of lone pairs
- Answer a
- Four lines are shown in this Lewis structure. Therefore, four bonds are present in this molecule. More specifically, this molecule contains two double bonds, because these four bonds are distributed such that two lines are located between the central carbon atom and each surrounding oxygen atom. Finally, there are four lone pairs, or pairs of dots, shown in this Lewis structure.
- Answer b
- Four lines are shown in this Lewis structure. Therefore, four bonds are present in this molecule. More specifically, this molecule contains one triple bond, which corresponds to the set of three lines located between the carbon atom and the nitrogen atom, and one single bond, which is found between the carbon atom and the hydrogen atom. Finally, there is one lone pair, or pair of dots, shown in this Lewis structure.
- Answer c
- Five lines are shown in this Lewis structure. Therefore, five bonds are present in this molecule. More specifically, this molecule contains one double bond, which corresponds to the set of two lines located between the carbon atom and the nitrogen atom, and three single bonds, which are found between each of the remaining pairs of atoms. Finally, there is one lone pair, or pair of dots, shown in this Lewis structure.
- Answer d
- Eight lines are shown in this Lewis structure. Therefore, eight bonds are present in this molecule. More specifically, this molecule contains one triple bond, which corresponds to the set of three lines located between the left-most and central carbon atoms, one double bond, which is located between the right-most carbon atom and the oxygen atom, and three single bonds, which are found between each of the remaining pairs of atoms. Finally, there are two lone pairs, or pairs of dots, shown in this Lewis structure.
- Answer e
- Eight lines are shown in this Lewis structure. Therefore, eight bonds are present in this molecule. More specifically, this molecule contains two double bonds, which are located between the central sulfur atom and the left-most and right-most surrounding oxygen atoms, and four single bonds, which are found between each of the remaining pairs of atoms. Finally, there are eight lone pairs, or pair of dots, shown in this Lewis structure.

