3.12: Ionic Bonding: Writing Chemical Formulas of Ionic Compounds Containing Polyatomic Ions
- Page ID
- 215710
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Write the chemical formulas of ionic compounds containing polyatomic ions.
Several common polyatomic ions, which have defined formulas, names, and charges that cannot be modified in any way, were introduced in Section 3.11. These charged units can form ionic bonds with oppositely-charged ions, which can be either monatomic or polyatomic. The processes for writing the chemical formula and the chemical name of an ionic compound containing a polyatomic ion will be presented and applied in the current and following sections of this chapter, respectively.
Writing Chemical Formulas of Ionic Compounds Containing Polyatomic Ions
The procedure for determining the chemical formula of an ionic compound containing exclusively main group elements or a combination of main group and transition metal elements can also be utilized to establish the chemical formula of an ionic compound that contains a polyatomic ion.
For example, consider the cyanide ion and beryllium.
Based on the combinations listed in Section 3.2, the cyanide ion and beryllium will combine to form an ionic compound. Recall that an ionic bond is produced when a cation exists in close physical proximity to an anion, creating an electrostatic attractive force. In the given combination, the cyanide ion is classified as a polyatomic anion, and beryllium, a metal, ionizes to form a cation. After establishing that a pair of chemicals will form an ionic bond, a five-step process can be employed to determine the chemical formula of the resultant ionic compound.
- Write the ion symbols for any indicated polyatomic ions and for any monatomic ion that results upon the ionization of a main group or transition metal element. Based on the information presented in the previous sections of this chapter, the cyanide ion is symbolized as CN–1, and beryllium ionizes to form Be+2.
- In order to ensure consistent formatting in all ionic chemical formulas, the symbol for the cation is written first. In this example, Be+2 will be written before CN–1 in the final chemical formula.
- The signs of the ions are only used to determine the relative order in which the ion symbols are written. As this information was established in the previous step, the "+" and "–" signs can be removed, so that only the base elemental symbols and numerical superscripts remain. In this example, the "+" sign is removed from Be+2 and the "–" sign is removed from CN–1. The revised symbols are written as Be2 and CN1, respectively.
- Use subscripts to indicate how many of each of the given ions are present in the base chemical formula. Two different processes, both of which are presented below, can be used to determine this information.
- The first process, which is considered the more "scientifically-correct" method, is known as the "Ratio Method," as it establishes the correct cation-to-anion ratio by equating the total charges of the cations to the sum of the charges of the anions, in order to ensure that the final compound will be a net-neutral species. To determine this ratio, a mini-equation, in which the larger superscript value is multiplied by 1, and the smaller superscript value is multiplied by a variable, such as x, is solved. In the current example, the larger superscript value is "2," and the smaller superscript value is "1." Therefore,
2(1) = 1(x)
This result indicates that for every 1 of the ions with the larger superscript value, in this case, Be2, 2 of the other ions, CN1, are required to achieve charge-balance between them.
2 = x
To apply this information, remove the numerical superscripts from each symbol and instead utilize subscripts to indicate the ratio established above. Completion of this step results in a base formula of Be1CN2. However, this formula does not accurately reflect the intended meaning of the second subscript. As written, the "2" indicates that 2 nitrogens are present, but the base formula should actually contain 2 cyanide ions. Because a polyatomic ion is an indivisible unit, its chemical formula must be enclosed inside of two parentheses when incorporated into an ionic chemical formula. The subscript that specifies how many of that ion are present within a compound must be written after the closing parenthesis. A subscript in this position refers to the entire polyatomic ion, not simply the last element that it contains, which accurately reflects its intended meaning. Additionally, a subscript that is written outside of a parenthetical unit does not impact the identity of the polyatomic ion, which cannot be modified, by definition. Therefore, the true base formula for this ionic compound is Be1(CN)2.
- The alternative process for establishing this chemical formula is a "shortcut" known as the "Criss-Cross Method." In this system, the superscript on the first symbol becomes the subscript on the second symbol, and the superscript on the second symbol is repositioned as the subscript on the first symbol, as shown below.
This "shortcut" is known as the "Criss-Cross Method" because the numerical values effectively "criss-cross" over one another when they are moved to their new positions. However, this formula does not accurately reflect the intended meaning of the second subscript. As written, the "2" indicates that 2 nitrogens are present, but the base formula should actually contain 2 cyanide ions. Because a polyatomic ion is an indivisible unit, its chemical formula must be enclosed inside of two parentheses when incorporated into an ionic chemical formula. The subscript that specifies how many of that ion are present within a compound must be written after the closing parenthesis. A subscript in this position refers to the entire polyatomic ion, not simply the last element that it contains, which accurately reflects its intended meaning. Additionally, a subscript that is written outside of a parenthetical unit does not impact the identity of the polyatomic ion, which cannot be modified, by definition. Therefore, the true base formula for this ionic compound is Be1(CN)2.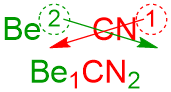
- The first process, which is considered the more "scientifically-correct" method, is known as the "Ratio Method," as it establishes the correct cation-to-anion ratio by equating the total charges of the cations to the sum of the charges of the anions, in order to ensure that the final compound will be a net-neutral species. To determine this ratio, a mini-equation, in which the larger superscript value is multiplied by 1, and the smaller superscript value is multiplied by a variable, such as x, is solved. In the current example, the larger superscript value is "2," and the smaller superscript value is "1." Therefore,
- The final step in this process is to ensure that the subscripts in the base formula are mathematically-appropriate for inclusion in an ionic compound.
- If possible, the subscripts that are written outside of the parentheses must be reduced to the lowest-common ratio of whole numbers by dividing both of the subscripts by the same numerical value. Any subscripts that are written inside of the parentheses may not be modified in any way, as those subscripts are defined by and, therefore, are integral to, the identity of the polyatomic ion. This division should only be performed if both of the resultant subscripts remain whole numbers. In the current example, no such division is possible. Therefore, the chemical formula remains unchanged: Be1(CN)2.
- If a "1" has been explicitly-written as a subscript, it must be removed. As indicated previously, values of "1" are usually implicitly-understood in chemistry and, therefore, should not be written in a chemical formula. In this example, the formula shown above does include an explicitly-written "1" and, therefore, should be revised to Be(CN)2.
The chemical formula that results upon the completion of this final step is a chemically-correct formula for an ionic compound. Therefore, Be(CN)2 is the chemical formula for the compound formed when the cyanide ion and beryllium bond with one another.

