2.1: Classifications of Matter
- Page ID
- 213145
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Define matter.
- Explain the difference between a pure substance and a mixture.
- Explain the difference between an element and a compound.
- Explain the difference between a homogeneous mixture and a heterogeneous mixture.
- Classify a substance as an element, a compound, a homogeneous mixture, or a heterogeneous mixture.
Matter is anything that has mass and volume. Nearly everything within the known universe, from water, to a fish, to the planets, is composed of matter. It may seem beneficial to have one vocabulary term to describe every substance that exists. However, each of these "things" has its own distinct characteristics and, therefore, can be classified using more specific terminology. Matter can be classified as either a pure substance or a mixture, based on its composition.
Pure Substances
A pure substance is a form of matter that has a definite composition. Furthermore, the physical properties, such as color, shine, density, and melting point, of a pure substance must be consistent throughout the entire sample. Pure substances can be categorized as either elements or compounds.
Elements
An element consists of only one type of atom and cannot be broken down into smaller pieces without changing its properties. Aluminum, which is found in soda cans, is an element. Helium, which is used to inflate balloons, is also an element. Carbon is an element that is essential to life on earth, as it is found in all living systems. "Aluminum," "helium," and "carbon" are all examples of elemental names. While many elemental names are short and (relatively) easy to spell, some, like "praseodymium" or "darmstadtium" are quite long and complex. Therefore, every elemental name has a corresponding abbreviation, called an elemental symbol. As was the case with the physical properties of chemistry that were defined in the previous chapter, specific rules must be followed when writing elemental symbols. While a complete elemental name can be written with its first letter being either capitalized or lower-case, the first letter in an elemental symbol must be capitalized, and any additional letters must be lower-case. Finally, note that some elemental symbols do not seem to match with their elemental name. These seemingly-mismatched elemental symbols actually correspond to a Greek or Latin word that can be used to describe the element. For example, "Hg" is the elemental symbol for the element named "mercury," which contains neither an "h" nor an "g" in its spelling! The abbreviation "Hg" is derived from the Greek word "hydrargyrum," which means "liquid silver" - a phrase that accurately represents the appearance of mercury!
Compounds
A compound or molecule must contain two or more elements that are combined in a specific ratio. Much like elements, each compound has a corresponding chemical name, which can be abbreviated using a chemical formula. The derivation of chemical formulas and chemical names will be the focus of the following chapter. A few simple examples will be mentioned here. "Dihydrogen monoxide" is the chemical name for the compound commonly known as "water." The chemical formula for this incredibly important substance is "H2O". Note that chemical names consist of words that resemble elemental names, and chemical formulas contain elemental symbols. The chemical formula for water contains an "H," which is the elemental symbol for "hydrogen," and an "O," which is the elemental symbol for "oxygen." Whole-number subscripts indicate how many of the previous element are present in the compound. If no subscript is written, an unwritten "1" is understood, as was the case for several of the numerical quantities that were discussed in the previous chapter. Therefore, water contains two hydrogens for every one oxygen.
Changing either the elements that are present or their ratio changes the identity of the compound and, therefore, its properties. For example, water (H2O) is used to extinguish fires, but dihydrogen sulfide (H2S) is highly flammable! Both of these chemicals contain two hydrogens, but exchanging the oxygen for a sulfur results in a chemical that behaves very differently. While water contains two hydrogens for every one oxygen, hydrogen peroxide (H2O2) contains a two-to-two ratio of hydrogen to oxygen. Again, the properties of these chemicals are very different: Water is safe to drink, but hydrogen peroxide, which is commonly used as a mild antiseptic, is definitely not!
Classify each of the following pure substances as an element or a compound.
- NaCl
- Co
- CO
Solutions
Answer a: Compound
Explanation: The capitalization of letters within a chemical formula can be used to divide that formula into unique elemental symbols. Since the "a" is lower-case, it must be the second letter of the elemental symbol "Na" (sodium). The Capitalized "C" indicates the start of a second elemental symbol. Since the "l" is lower-case, it must be the second letter of that second elemental symbol, Cl (chlorine). Since two elements are present, this is a compound (sodium chloride, which is more commonly-known as "table salt.") Note that the elements are present in a one-to-one ratio, as neither elemental symbol has an explicitly-written subscript.
Answer b: Element
Explanation: Since the "o" is lower-case, it must be the second letter of the elemental symbol beginning with a capital "C". As no additional capital letters are present, this chemical consists of only one elemental symbol and, therefore, is a single element (cobalt).
Answer c: Compound
Explanation: Since both the "C" and the "O" are capitalized, each must represent its own unique element (carbon and oxygen, respectively). Since two elements are present, this is a compound (carbon monoxide). Again, the elements are present in a one-to-one ratio, as neither elemental symbol has an explicitly-written subscript.
Mixtures
A mixture is a blend of two or more elements or compounds, which have their own identities and properties. Mixtures, which can be homogeneous or heterogeneous, can be separated into their individual components because the substances are not chemically-connected or bonded to one another.
Homogeneous Mixtures
A homogeneous mixture has a uniform composition, meaning that if several samples are taken, each would contain the same chemicals in approximately the same ratio. Salt water is an example of a homogeneous mixture, as it contains two distinctive chemicals, a "salt," which is most likely sodium chloride, NaCl, and water, H2O. These compounds can be separated by boiling the water, leaving the salt as a residue. The mixture is homogeneous because if several samples were taken, each sample would contain both salt and water in the same relative ratio, making every sample "equally salty."
Heterogeneous Mixtures
A heterogeneous mixture has a non-uniform composition, meaning that if several samples are taken, each would contain either completely different chemicals or the same chemicals, but in different ratios. A chocolate chip cookie is a heterogeneous mixture, because it contains many different chemicals that form the cookie dough and the chocolate chips, which can be separated by pulling out the chips. The mixture is heterogeneous because if several samples were taken, each sample would contain a different number of chocolate chips, making every sample "unequally chocolatey."
Classify each of the following mixtures as being homogeneous or heterogeneous.
- Vegetable soup
- 31 Cents (a quarter, a nickel, and a penny)
- Wine
Solutions
Answer a: Heterogeneous
Explanation: The vegetables present in a vegetable soup are not uniformly-distributed. Therefore, every "sample" taken when eating the soup is different.
Answer b: Heterogeneous
Explanation: Each of these coins is uniquely-sized and -shaped, making the mixture non-uniform.
Answer c: Homogeneous
Explanation: Wines and other liquors contain ethanol, which is the chemical commonly-known as "drinking alcohol," water, and different flavorings. These individual components are uniformly blended with one another, so every "sample" taken when drinking a glass of wine would have the same color, aroma, and flavor.
Summary
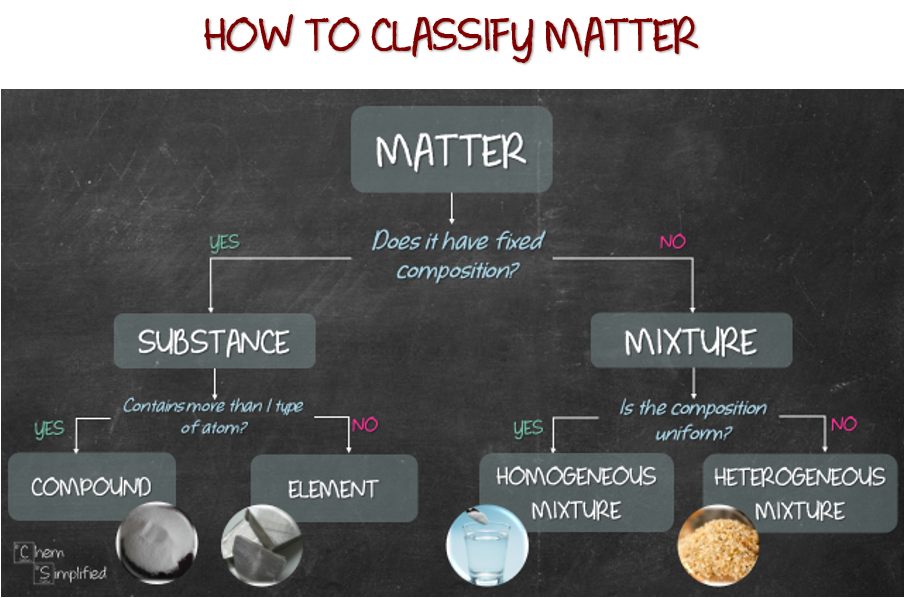
The vocabulary discussed in this section is summarized below in Figure \(\PageIndex{1}\). This image also includes simple "yes-or-no" tests that can be used to help distinguish a pure substance from a mixture, an element from a compound, and a homogeneous mixture from a heterogeneous mixture.
Classify each of the following substances as an element, a compound, a homogeneous mixture, or a heterogeneous mixture.
- Filtered coffee
- Neon (Ne)
- Spaghetti and meatballs
- Sucrose, which is commonly-known as "table sugar" (C12H22O11)
- Answer a
- Answer Homogeneous mixture
Explanation: Coffee contains a variety of chemicals that are dissolved in water during the brewing process. Since multiple different chemicals are present, coffee is a mixture. Because the coffee is described as "filtered," any particles that do not dissolve have been removed, and the remaining drink would have a uniform composition. (Every "sample" taken when drinking a cup of coffee would have the same color, aroma, and flavor.) - Answer b
- Answer Element
Explanation: Neon is symbolized using a single elemental symbol, "Ne". Therefore, neon is an element. - Answer c
- Answer Heterogeneous mixture
Explanation: Spaghetti and meatballs contains many different chemicals that form the noodles, the sauce, and the meatballs, which can all be separated from one another. Therefore, spaghetti and meatballs is a mixture. Since every "sample" eaten would have different amounts of each of these ingredients, the overall composition of this dish is non-uniform. - Answer d
- Answer Compound
Explanation: Since the "C," the "H," and the "O" are all capitalized in the given formula, each must represent its own unique element (carbon, hydrogen, and oxygen, respectively). Since multiple elements are present in a single chemical formula, this is a compound. (The elements are present in a 12-to-22-to-11 ratio, based on the subscripts in the chemical formula.)

