7.6: Solution Equations: Strong Electrolytes
- Page ID
- 222255
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
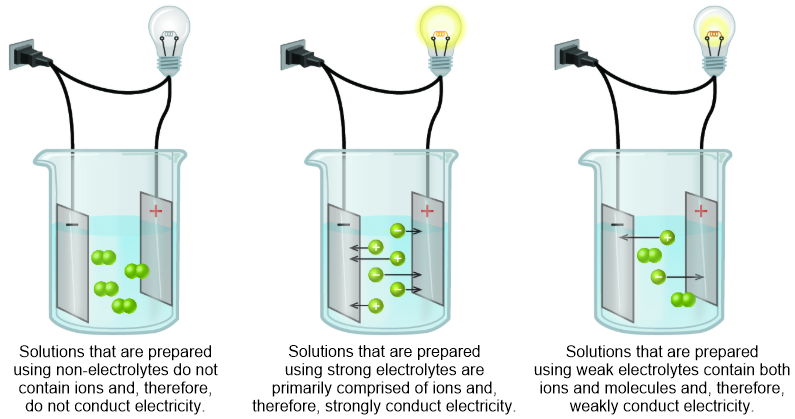
A solute that completely dissociates, or separates, into its constituent cations and anions during the solvation process is classified as a strong electrolyte. Because an electrical current can flow between the resultant ions, solutions that are generated through the solvation of strong electrolytes will strongly conduct an electrical current, as illustrated in the second image that is shown in Figure \(\PageIndex{1}\).
The dissociative behavior that is exhibited by a strong electrolyte is represented in the solution equation pattern that is shown below. Although a chemical change does not occur when a solution is formed, a "reaction" arrow, "\(\rightarrow\)," which can also be called a yield sign, is used in a solution equation to indicate that solvation and, therefore, a physical change, has occurred. Because a solution equation is the symbolic representation of a physical change, the chemical formulas, not the chemical names, of the substances that are being transformed and created are incorporated into the pattern that is shown below. Since a solution equation is used to represent the electrolyte behavior of the chemical that is being dissolved, the chemical formula of the solute is written on the left side of the "reaction" arrow. A solution also contains a solvent, which does not undergo a physical change during the dissolving process and, therefore, does not exhibit any electrolyte behavior. As a result, the formula of the solvent should not be written on the left side of the "reaction" arrow. Instead, the chemical formula of the solvent is written over the "reaction" arrow, in order to indicate the presence of this substance in the resultant solution. Because a strong electrolyte completely dissociates, or separates, into its constituent cations and anions as it dissolves, an ion symbol, which indicates the charge of the ion as a superscript on the corresponding elemental symbol, for each of these particles is written on the right side of the "reaction" arrow. Since both cations and anions, which can be monoatomic or polyatomic, are generated during the solvation process, a plus sign, "+", is used to separate their ion symbols.
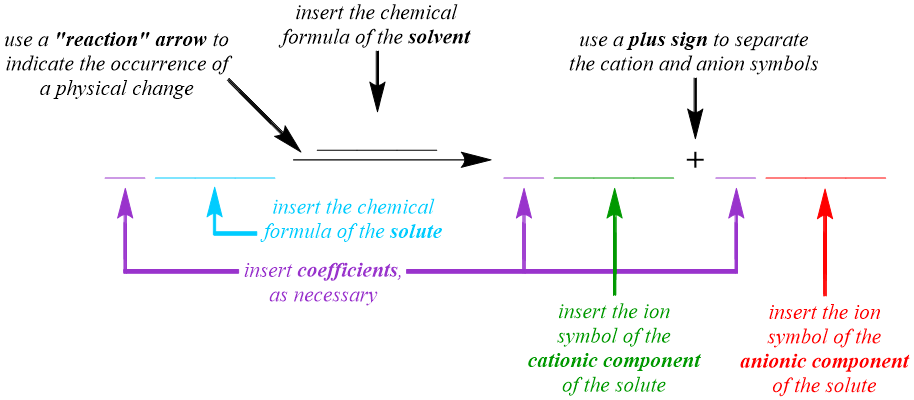
As stated in Section 7.2, solutions can be prepared using solvents and solutes that exist in the solid, liquid, or gaseous states of matter. If water is utilized to dissolve a solute, the substances that are present in the resultant solution are classified as aqueous, by definition. While these states of matter can be incorporated into a solution equation using the abbreviations "\(\left( s \right)\)," "\(\left( l \right)\)," "\(\left( g \right)\)," and "\(\left( aq \right)\)," respectively, the information that is conveyed by these symbols is not vital to understanding the electrolyte behavior of the solute and, therefore, the states of matter are often omitted from solution equations.
Finally, most solution equations require the incorporation of one or more balancing coefficients, in order to indicate that the Law of Conservation of Matter, which mandates that particles cannot be created or destroyed during a physical or chemical change, is upheld during the solvation process. Recall that a coefficient is a whole-number value that specifies the quantity in which the corresponding particle participates in the transformation that is occurring, and that values of "1" are usually implicitly-understood in chemistry and, therefore, are not written when balancing an equation. As stated above, the solvent does not undergo a physical change during the dissolving process and, consequently, does not exhibit any electrolyte behavior. Therefore, because coefficients are only associated with chemicals that are changing, the chemical formula of the solvent is not considered when balancing a solution equation.

