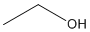Chemistry is the experimental and theoretical study of materials on their properties at both the macroscopic and microscopic levels. Understanding the relationship between properties and structures/bonding is also a hot pursuit. Chemistry is traditionally divided into organic and inorganic chemistry. The former is the study of compounds containing at least one carbon-hydrogen bonds. By default, the chemical study of all other substances is called inorganic chemistry, a less well defined subject.
However, the boundary between organic and inorganic compounds is not always well defined. For example, oxalic acid, H2C2O4, is a compound formed in plants, and it is generally considered an organic acid, but it does not contain any C-H bond. Inorganic chemistry is also closely related to other disciplines such as materials sciences, physical chemistry, thermodynamics, earth sciences, mineralogy, crystallography, spectroscopy etc.
A chemical formula is a format used to express the structure of atoms. The formula tells which elements and how many of each element are present in a compound. Formulas are written using the elemental symbol of each atom and a subscript to denote the number of elements. This notation can be accredited to Swedish chemist Jons Jakob Berzeliu. The most common elements present in organic compounds are carbon, hydrogen, oxygen, and nitrogen. With carbon and hydrogen present, other elements, such as phosphorous, sulfur, silicon, and the halogens, may exist in organic compounds. Compounds that do not pertain to this rule are called inorganic compounds.
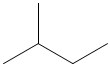
Understanding how atoms in a molecules are arranged and how they are bonded together is very important in giving the molecule its identity. Isomers are compounds in which two molecules can have the same number of atoms, and thus the same molecular formula, but can have completely different physical and chemical properties because of differences in structural formula.

Methylpropane and butane have the same molecular formula of C4H10, but are structurally different (methylpropane on the left, butane on the right).
Polymers
A polymer is formed when small molecules of identical structure, monomers, combine into a large cluster. The monomers are joined together by covalent bonds. When monomers repeat and bind, they form a polymer. While they can be comprised of natural or synthetic molecules, polymers often include plastics and rubber. When a molecule has more than one of these polymers, square parenthesis are used to show that all the elements within the polymer are multiplied by the subscript outside of the parenthesis. The subscript (shown as n in the example below) denotes the number of monomers present in the macromolecule (or polymer).
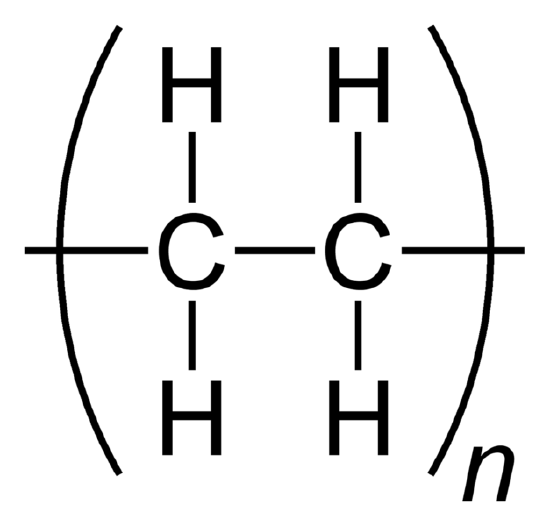
Ethylene becomes the polymer polyethylene.
The molecular formula is based on the actual makeup of the compound. Although the molecular formula can sometimes be the same as the empirical formula, molecular compounds tend to be more helpful. However, they do not describe how the atoms are put together. Molecular compounds are also misleading when dealing with isomers, which have the same number and types of atoms (see above in molecular geometry and structural formula).
Ex. Molecular Formula for Ethanol: C2H6O.
An empirical formula shows the most basic form of a compound. Empirical formulas show the number of atoms of each element in a compound in the most simplified state using whole numbers. Empirical formulas tend to tell us very little about a compound because one cannot determine the structure, shape, or properties of the compound without knowing the molecular formula. Usefulness of the empirical formula is decreased because many chemical compounds can have the same empirical formula.
Ex. Find the empirical formula for C8H16O2.
Answer: C4H8O (divide all subscripts by 2 to get the smallest, whole number ratio).
A structural formula displays the atoms of the molecule in the order they are bonded. It also depicts how the atoms are bonded to one another, for example single, double, and triple covalent bond. Covalent bonds are shown using lines. The number of dashes indicate whether the bond is a single, double, or triple covalent bond. Structural formulas are helpful because they explain the properties and structure of the compound which empirical and molecular formulas cannot always represent.
Ex. Structural Formula for Ethanol: 
Condensed structural formulas show the order of atoms like a structural formula but are written in a single line to save space and make it more convenient and faster to write out. Condensed structural formulas are also helpful when showing that a group of atoms is connected to a single atom in a compound. When this happens, parenthesis are used around the group of atoms to show they are together.
Ex. Condensed Structural Formula for Ethanol: CH3CH2OH (Molecular Formula for Ethanol C2H6O).
Because organic compounds can be complex at times, line-angle formulas are used to write carbon and hydrogen atoms more efficiently by replacing the letters with lines. A carbon atom is present wherever a line intersects another line. Hydrogen atoms are then assumed to complete each of carbon's four bonds. All other atoms that are connected to carbon atoms are written out. Line angle formulas help show structure and order of the atoms in a compound making the advantages and disadvantages similar to structural formulas.
Ex. Line-Angle Formula for Ethanol: 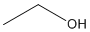
Inorganic compounds are typically not of biological origin. Inorganic compounds are made up of atoms connected using ionic bonds. These inorganic compounds can be binary compounds, binary acids, or polyatomic ions.
Binary compounds
Binary compounds are formed between two elements, either a metal paired with a nonmetal or two nonmetals paired together. When a metal is paired with a nonmetal, they form ionic compounds in which one is a negatively charged ion and the other is positvely charged. The net charge of the compound must then become neutral. Transition metals have different charges; therefore, it is important to specify what type of ion it is during the naming of the compound. When two nonmetals are paired together, the compound is a molecular compound. When writing out the formula, the element with a positive oxidation state is placed first.
Ex. Ionic Compound: BaBr2(Barium Bromide)
Ex. Molecular Compound: N2O4 (Dinitrogen Tetroxide)
Binary acids
Binary acids are binary compounds in which hydrogen bonds with a nonmetal forming an acid. However, there are exceptions such as NH3, which is a base. This is because it shows no tendency to produce a H+. Because hydrogen is positively charged, it is placed first when writing out these binary acids.
Ex. HBr (Hydrobromic Acid)
Polyatomic ions
Polyatomic ions is formed when two or more atoms are connected with covalent bonds. Cations are ions that have are postively charged, while anions are negatively charged ions. The most common polyatomic ions that exists are those of anions. The two main polyatomic cations are Ammonium and Mercury (I). Many polyatomic ions are typically paired with metals using ionic bonds to form chemical compounds.
Ex. MnO4- (Polyatomic ion); NaMnO4 (Chemical Compound)
Oxoacids
Many acids have three different elements to form ternary compounds. When one of those three elements is oxygen, the acid is known as a oxoacid. In other words, oxacids are compounds that contain hydrogen, oxgygen, and one other element.
Ex. HNO3 (Nitric Acid)
Complex Compounds
Certain compounds can appear in multiple forms yet mean the same thing. A common example is hydrates: water molecules bond to another compound or element. When this happens, a dot is shown between H2O and the other part of the compound. Because the H2O molecules are embedded within the compound, the compound is not necessarily "wet". When hydrates are heated, the water in the compound evaporates and the compound becomes anhydrous. These compounds can be used to attract water such as CoCl2. When CoCl2 is dry, CoCl2 is a blue color wherease the hexahydrate (written below) is pink in color.
Ex. CoCl2 ·6 H2O
Organic compounds contain a combination carbon and hydrogen or carbon and hydrogen with nitrogen and a few other elements, such as phosphorous, sulfur, silicon, and the halogens. Most organic compounds are seen in biological origin, as they are found in nature.
Hydrocarbons
Hydrocarbons are compounds that consist of only carbon and hydrogen atoms. Hydrocarbons that are bonded together with only single bonds are alkanes. The simplest example is methane (shown below). When hydrocarbons have one or more double bonds, they are called alkenes. The simplest alkene is Ethene (C2H4) which contains a double bond between the two carbon atoms.
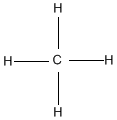
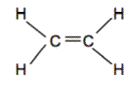
Ex. Methane on left, Ethene on right
Functional Groups
Functional groups are atoms connected to carbon chains or rings of organic molecules. Compounds that are within a functional group tend to have similar properties and characteristics. Two common functional groups are hydroxyl groups and carboxyl groups. Hydroxyl groups end in -OH and are alcohols. Carboxyl groups end in -COOH, making compounds containing -COOH carboxylic acids. Functional groups also help with nomenclature by using prefixes to help name the compounds that have similar chemical properties.
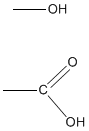
Ex. Hydroxyl Group on top; Carboxyl Group on bottom
References
- Miessler, Gary L. Inorganic Chemistry. 2nd. Upper Saddle River: Prentince Hall, 1999.
- Munowitz, Michael. Principles of Chemistry. Norton & Company: New York, 2000.
- Pettrucci, Ralph H. General Chemistry: Principles and Modern Applications. 9th. Upper Saddle River: Pearson Prentice Hall, 2007.
Problems
- Which of the following formulas are organic?
- HClO
- C5H10
- CO2
- What is the name of the following formula?
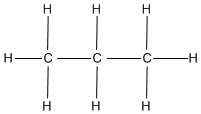
- Classify the following formulas into their appropriate functional group
- Acetic acid
- Butanol
- Oxalic acid
- What are the empirical formulas for the following compounds?
- C12H10O6
- CH3CH2CH2CH2CH2CH2CH3
- H3O
- What is the name of the following figure and what is the molecular formula of the following figure?
Answer Key:
1. b and c. 2. Propane. 3. a. carboxyl group, b. hydroxyl group, c. carboxyl group. 4. a. C6H5O3, b. C7H16, c. H3O. 5. Methylbutane, C5H12
Contributors and Attributions
- Jean Kim (UCD), Kristina Bonnett (UCD)