5.8: Using Nuclear Science to Diagnose Disease
- Page ID
- 85498
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Understand the difference between diagnostic and therapeutic radiation.
- Know what testing involve nuclear chemistry.
- Calculate dosages of radiation received with a nuclear test.
- Compare nuclear testing dosages to toxic concentrations.
Diagnostic medical applications involve testing for a disease or condition. In nuclear medicine, this could involve using x-rays, CT or PET scans, or isotopic studies. The radiation involved for each of these types of tools will vary in mrem or mSv amounts.
X-Ray Imaging
X-Ray imaging is one of the most basic and routine forms of imaging within modern medicine. Using electromagnetic radiation, scientists and doctors have the ability to visualize internal situations. X-Ray imaging takes advantage of the fact that dense structures (i.e., bone) absorb more x-rays than the softer tissues that surround it. By projecting radiation onto special film paper, the denser substance casts a shadow where less x-rays pass through the body, and a contrasting image is produced. The radiation emitted by an X-Ray is of the wavelength of 10 to 0.01 nanometers (nm). Because some parts of the body may be thicker than others, X-Rays are used in "soft" (10 to 0.10 nm) and "hard" (0.10 to 0.01 nm) dosages or radiation.
X-rays are produced by passing an electron beam through a tungsten anode in a vacuum. The electromagnetic waves that are released can be focused and adjusted according to what is imaged. X-Rays can image in very high definition at a low cost, but they provide poor contrast in soft tissues. Contrast agents can also be added to the body prior to imaging, which allows different tissues to absorb more radiation, creating a better contrasting image. This allows doctors and scientists to work with much more specific images within their diagnosis or research (i.e., the ability to determine different aspects of soft-tissue).
Projectional radioagraphy (Figure \(\PageIndex{1}\)) can be done standing or laying down. To obtain clearer images, a patient needs to be as still as possible. A technician may ask to take a big breath and hold it right before pushing the scan button. This allows the patient to keep their chest and abdomen in position and will allow a clearer image to be captured. Breathing will cause movement which will result in a blurred image.
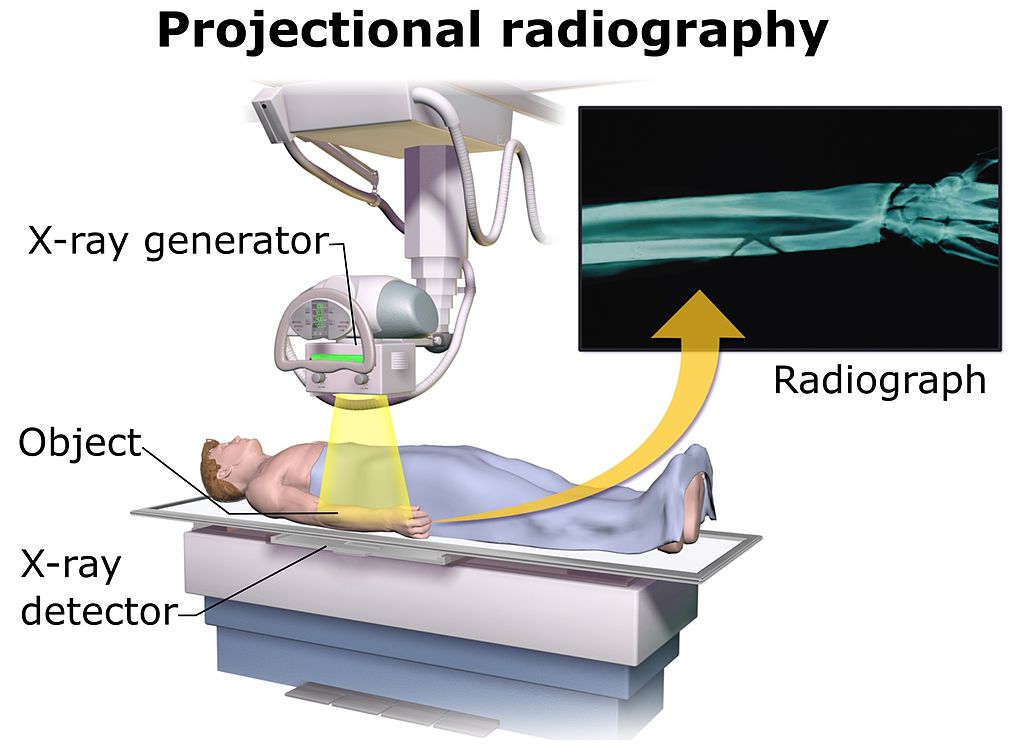
Before having an x-ray or CT scan, patients should remove all metallic clothing or jewelry. Metals can distort x-ray images easily. Sometimes, lead aprons are provided to patients. The lead aprons shields a patient's reproductive organs from ionizing radiation. X-rays and CT scans can harm a developing embryo or fetus. For this reason, female patients are required to sign a liability wavier stating they are not pregnant at the time of testing. Other options like MRI or ultrasounds (nonionzing techniques) are available for these patients.
Diagnostic x-rays differ greatly in millisievert (mSV) dosages. Table \(\PageIndex{1}\) illustrates a few of these values. Using this table, you can calculate rem and mrem amounts and compare it to toxic values shown in Section 5.6.
| Type of X-ray Exam | Diagnostic Reference level (DRL) in mSv |
|---|---|
| Chest | 0.96 |
| Abdomen | 7.10 |
| C-spine | 3.56 |
| T-sping | 8.00 |
| L-Spline | 12.0 |
| Skull | 6.00 |
| Lumb | 7.21 |
| Hip | 7.21 |
| Source: Sonawane AU1, Shirva VK, Pradhan AS., Radiat Prot Dosimetry. 2010 Feb;138(2):129-36. doi: 10.1093/rpd/ncp235. | |
A chest x-ray yields a dosage of 6 mrems of radiation to a patient. Calculate this dosage in rems and millisieverts (mSv). Compare these values to your annual background dose (around 360 mrems) and cancer dose (100 rems).
Solution
For the first calculation, recall the metric prefix milli from Chapter 2.
\[\begin{array}{c|c } 6\, \cancel{mrem} & 1\, rem \\ \hline 1 & 1000\, \cancel{mrem} \\ \end{array} = 6 \times 10^{-3} \,rem \nonumber \]
For the second calculation, you will need to insert the 100 rem = 1 Sv factor. Then, you will need to use your milli metric prefix again.
\[\begin{array}{c|c | c } 6 \times 10^{-3} \, \cancel{rem} & 1\, \cancel{Sv} & 1000 \, mSv\\ \hline 1 & 100\, \cancel{rem} & 1\, \cancel{Sv} \\ \end{array} = 6 \times 10^{-2} \,mSv \nonumber \]
The dose of 6 mrem dose is significantly lower than the level of background radiation. In addition, the calculated \(6\times 10^{-3} \,rem\) value is well below the 100 rem dosage that is associated with development of cancer.
Your grandmother/mother goes to get her yearly mammogram (x-ray image that helps detect breast cancer). The radiation level for this scan is .36mSv. Convert this value to mrem and rem. Compare calculated values to background and cancer causing levels (shown in previous problem).
\[\begin{array}{c|c | c } 0.36 \, \cancel{mSv} & 1\, \cancel{Sv} & 100 \, rem\\ \hline 1 & 1000\, \cancel{mSv} & 1\, \cancel{Sv} \\ \end{array} = 3.6 \times 10^{-2} \,rem \nonumber \]
\[\begin{array}{c|c } 3.6 \times 10^{-2}\, \cancel{rem} & 1000\, mrem \\ \hline 1 & 1\, \cancel{rem} \\ \end{array} = 36 \,mrem\nonumber \]
36 mrems is less than an average person's background radiation of 360 mrems. In addition, this value is 96.4 rems below the cancer level of 100 rems.
CT Scanning
Imagine a patient enters the hospital complaining of severe chest pain. After consulting the patient, the doctors decide that the source of the patient's problems is a constricted artery. However, the human body contains several long arteries. The doctors could use surgery to find the source of the clot, but this could take many hours and with no definite guarantee of success. This is exactly where radiation helps medical professionals bridge the gap that once existed. Computerized Axial Tomography (CT) scans use X-ray particles along with advanced computer technology in order to produce highly detailed cross sectional images of the body. Using these scans, medical professionals can accurately find clots and other other harmful medical conditions.
CT/CAT scans have the same fundamentals of x-rays, but they produce 3-D images using tomography. Using digital geometric processing, the CT scan creates a 3-D image compiling a large number of 2-D x-rays. These 2-D images are thin cross sections of the body taken from a single axis of rotation. A CT scanner consists of a table where the patient lies on, an x-ray device which rotates at high speed around the patient taking hundreds of cross sections of the body, and a computer with appropriate software to compile and render the 3-D image. The table in Figure 1 moves through the tube, allowing the machine to take hundreds of pictures from head to toe.
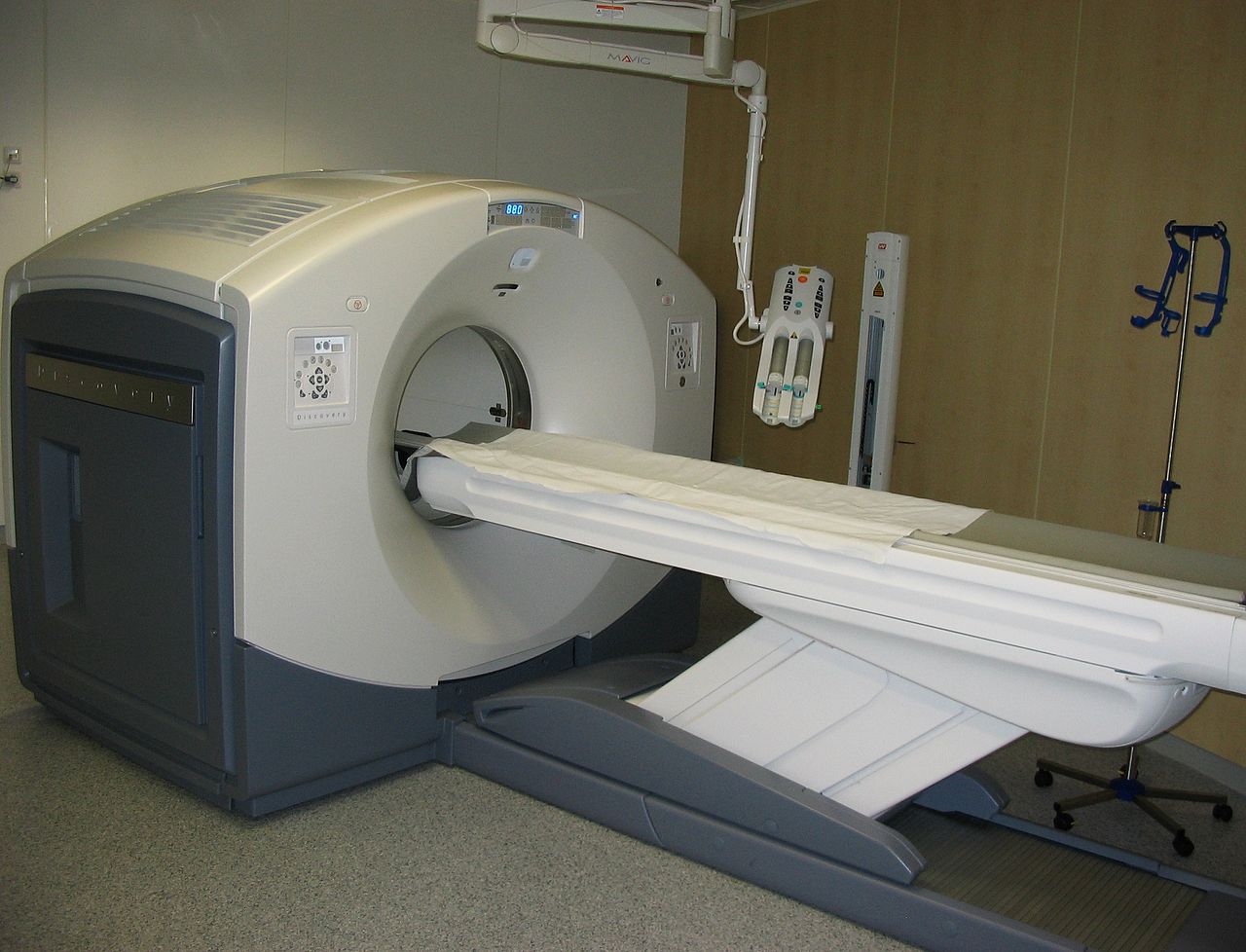
Similar to x-ray, contrast agents are typically added to the body before imaging occurs so that different tissues are more defined. Due to the fact that many more images are being taken in order to form a complete three dimensional image, the radiation dose is much higher. While a chest x-ray is around 0.10 mSv or radiation, a chest CAT scan subjects the patient to about 8 mSv of radiation; over twice the amount that the body would absorb from its environment in a year. For this reason, it is uncommon and unsafe to have multiple CAT scans taken unless completely necessary.
Your doctor suspects you have appendicitis and orders a CT scan. This type of evaluation exposes you to 12 msV of radiation. Convert the given mSv amount to rem and mrem. Lastly, compare this value to background and cancer mrem/rem values.
\[\begin{array}{c|c | c } 12\, \cancel{mSr} & 1\, \cancel{Sr} & 100 \, rem \\ \hline 1 & 1000\, \cancel{mSr} & 1\, \cancel{Sr} \\ \end{array} = 1.2 \,rem \nonumber \]
\[\begin{array}{c|c } 1.2\, \cancel{rem} & 1000\, mrem \\ \hline 1 & 1\, \cancel{rem} \\ \end{array} = 1200 \,mrem \nonumber \]
PET Scanning
Positron Emission Tomography or PET scan is a type of nuclear medicine imaging. Depending on the area of the body being imaged, a radioactive isotope is either injected into a vein, swallowed by mouth, or inhaled as a gas. When the radioisotope is collected in the appropriate area of the body, the gamma ray emissions are detected by a PET scanner (often called a gamma camera) which works together with a computer to generate special pictures, providing details on both the structure and function of various organs. Watch this informational video on how this technique works. PET scans are used to:
- Detect cancer
- Determine the amount of cancer spread
- Assess the effectiveness of treatment plans
- Determine blood flow to the heart muscle
- Determine the effects of a heart attack
- Evaluate brain abnormalities such as tumors and memory disorders
- Map brain and heart function
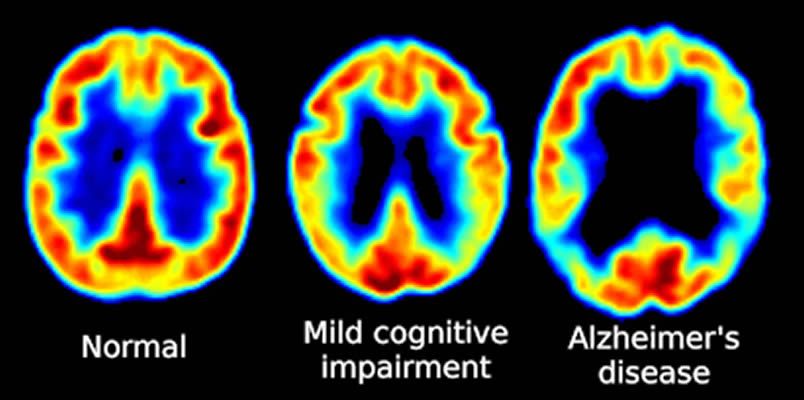
PET Scanning, is used to image the physiological aspects of the body rather than the anatomy. It images the function of the body rather than the form, such as where tagged molecules go and how they are used. For instance, if you were to image the brain of a deceased person, nothing would show up on a PET scan opposed to a CAT scan, as the brain is no longer functional. Pet Scanning is very useful in imaging tumors, which can be done when patients are injected with certain tracers. Often times PET scanners are used in collaboration with CAT scanners to create a composite image that shows both the function and form of the body. The animation below is a whole-body PET scan using the radioisotope of 18F (t1/2 = 110 min). Using this tracer, doctors can determine if cancer has metastasized by looking at the metabolic activity of glucose.
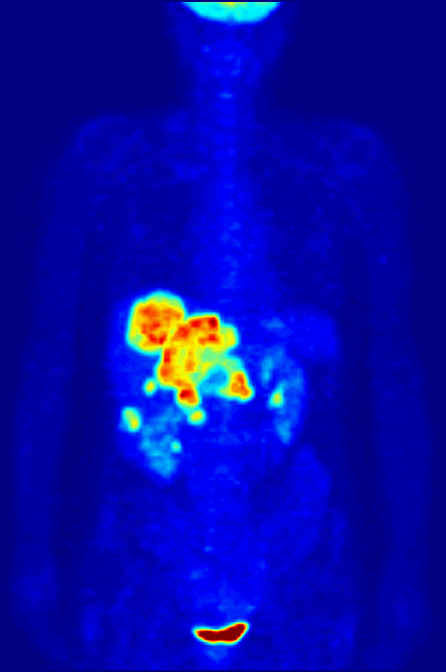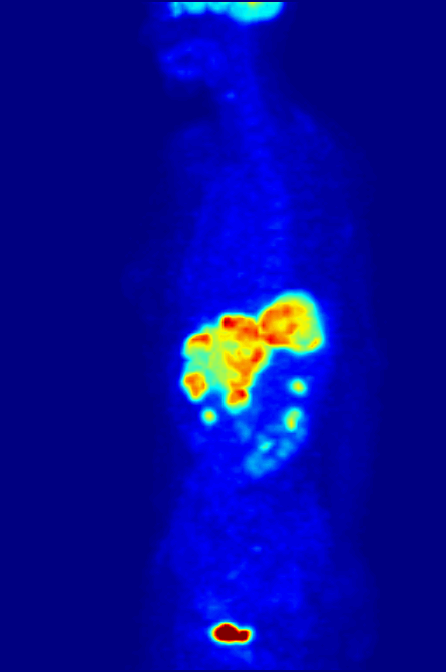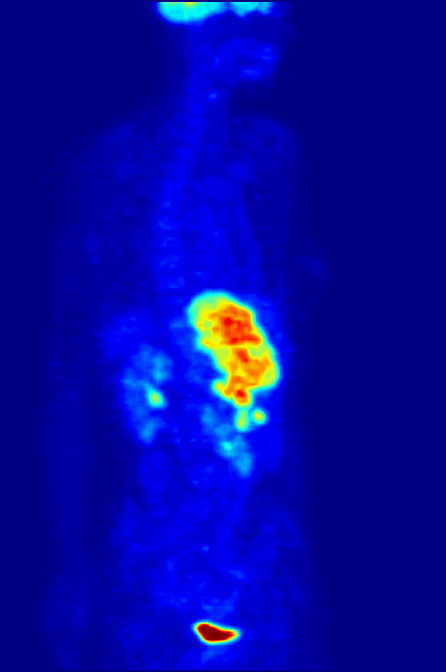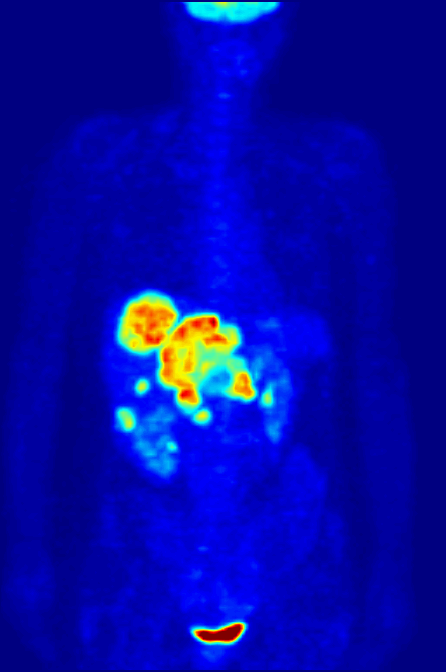
Other Isotopic Tests
Radioisotopes have revolutionized medical practice, where they are used extensively. More than 10 million nuclear medicine procedures and more than 100 million nuclear medicine tests are performed annually in the United States. Four typical examples of radioactive tracers used in medicine are technetium-99 \(\ce{(^{99}_{43}Tc)}\), thallium-201 \(\ce{(^{201}_{81}Tl)}\), iodine-131 \(\ce{(^{131}_{53}I)}\), and sodium-24 \(\ce{(^{24}_{11}Na)}\). Damaged tissues in the heart, liver, and lungs absorb certain compounds of technetium-99 preferentially. After it is injected, the location of the technetium compound, and hence the damaged tissue, can be determined by detecting the γ rays emitted by the Tc-99 isotope. Thallium-201 (Figure \(\PageIndex{1}\)) becomes concentrated in healthy heart tissue, so the two isotopes, Tc-99 and Tl-201, are used together to study heart tissue. Iodine-131 concentrates in the thyroid gland, the liver, and some parts of the brain. It can therefore be used to monitor goiter and treat thyroid conditions, such as Grave’s disease, as well as liver and brain tumors. Salt solutions containing compounds of sodium-24 are injected into the bloodstream to help locate obstructions to the flow of blood.
Small doses of \(\ce{I}\)-131 (too small to kill cells) are used for purposes of imaging the thyroid. Once the iodine is concentrated in the thyroid, the patient lays down on a sheet of film and the radiation from the \(\ce{I}\)-131 makes a picture of the thyroid on the film. The half-life of iodine-131 is approximately 8 days so after a few weeks, virtually all of the radioactive iodine is out of the patient's system. During that time, they are advised that they will set off radiation detectors in airports and will need to get special permission to fly on commercial flights.
Some isotopes that are used to diagnose diseases are shown in Table \(\PageIndex{2}\). Be sure to memorize one symbol-mass format and application for the test on this unit. All of these nuclear isotopes release one form of ionizing radiation (either/add particle or ray). In addition, each isotopic application would involve a specific amount of mrem/mSv radiation.
| Symbol-mass | Half-Life (t1/2) | Application |
|---|---|---|
| Xe-133 | 5.27 days | Lung imaging |
| H-3 | 12.26 years | Analyzing total body water |
| Tl-201 | 73 hours | Stress tests for heart problems |
| Fe-59 | 44.5 days | Detection of anemia |
| Gd-153 | 242 days | Analyzing bone density |
| Cr-51 | 27.8 days | Determining blood volume |
| C-11 | 20.4 minutes | Brain scans |
| Tc-99m | 6.0 hours | Heart, lung, kidney, bone marrow, brain, or bone marrow imaging |
| Pu-238 | 86 years | Powering pacemakers |
| I-131 | 8.0 days | Imaging Thyroid |
Keep in mind that x-rays, CT scans, PET scans, and isotopic studies involve ionizing radiation. In contrast, MRI (magnetic resonance imaging) and ultrasounds do not utilize ionizing forms of radiation.
I like this picture-www.reddit.com/r/coolguides/comments/cv5mwx/brain_imaging/
Need more practice?
Refer to conversion worksheets on moodle.
Sources
- http://cerncourier.com/cws/article/cern/66177 (radioisotope information)
- en.Wikipedia.org/wiki/Nuclear_medicine (radioisotope information)
Contributors and Attributions
- Mike Reed (UC Davis), Eugene Kwon (UC Davis)
- Emma Gibney (Furman University)


