6.4: The Manhattan Project - Critical Mass and Bomb Construction
- Page ID
- 106760
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain the concept of critical mass for a fission reaction.
- Know the bomb mechanisms of Little Boy and Fat Man.
Determining Critical Mass
Materials that can sustain a nuclear fission chain reaction is said to be fissile or fissionable. This reaction becomes self-sustaining when the number of neutrons produced by fission equals or exceeds the number of neutrons absorbed by splitting nuclei plus the number that escape into the surroundings. The amount of a fissionable material that will support a self-sustaining chain reaction is a critical mass.
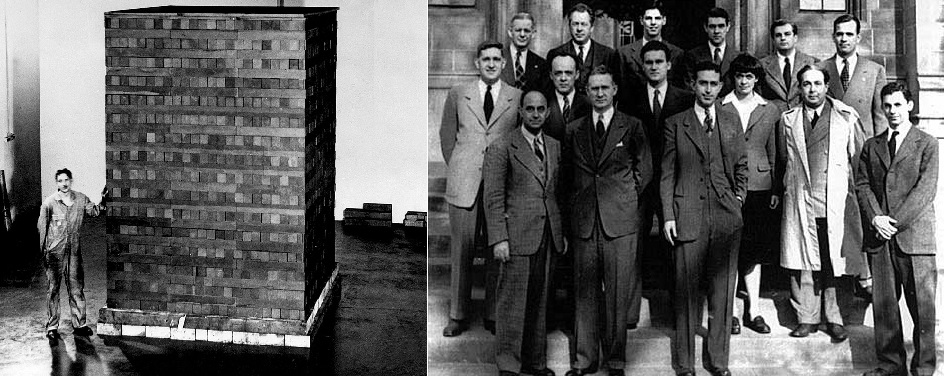
In order to determine critical mass required for a nuclear chain reaction, Enrico Fermi and Leo Szilard constructed the world's first controlled fission experiment. At the University of Chicago, the physicists piled 40,000 graphite (carbon based pencil material) blocks in a 24 square foot formation (see below). Fermi and Szilard embedded the graphite with 19,000 pieces of uranium. In a squash court (similar to a racketball court) on December 2, 1942, slow neutrons bombarded the pile to produce a sustained, nuclear chain reaction. The fission reaction was detectable by using a Geiger Counter. This mini reactor did not explode or injure any scientists due to its design. From these experiments, Fermi, Szilard and their colleagues determined the critical mass of U-235 to be approximately 50 kg. In addition, they concluded that critical mass was dependent upon the type of fuel ( U-235 or Pu-239), shape of fuel (round reduces amount), temperature of the reaction (higher increased neutron collisions), and density of material. Other experiments and calculations lead to the critical mass determination of Pu-239. Unlike U-235, only 10 kg of Pu-239 was required to sustain a nuclear chain reaction.

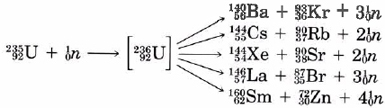
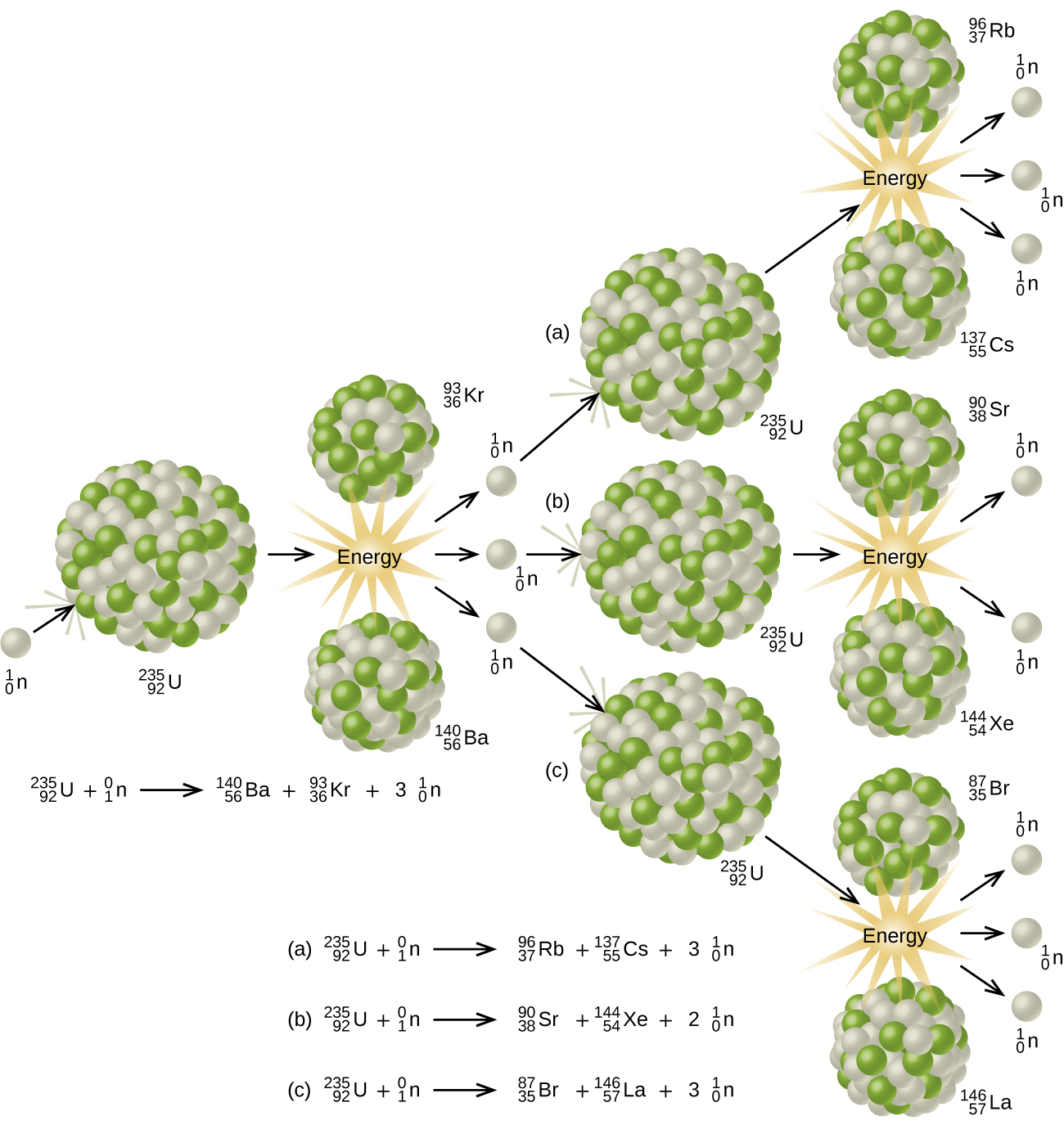
The fission of uranium produces more neutrons than it consumes. As can be seen from Eqs. (1), for every neutron captured by a U-235 nucleus, between two and four neutrons are produced. As soon as a stray neutron bombards a U-235 nucleus, fission will take place and three neutrons will be produced. These in turn will fission three more U-235 nuclei, producing a total of nine neutrons. A third repetition will produce 27 neutrons. a fourth 81. and so on. This process (which is called a chain reaction) escalates very rapidly. Within a few microseconds a very large number of nuclei fission, with the release of a tremendous amount of energy, and an atomic explosion results.
There are two reasons why a normal sample of uranium metal does not spontaneously explode in this way. Natural uranium consists mainly of the isotope U-238 while the fissionable isotope U-235 comprises only 0.7 percent of the total. Most of the neutrons produced in a given fission process are captured by U-238 nuclei without any further production of neutrons. The escalation of the fission process thus becomes impossible. However, even a sample of pure U-235 will not always explode spontaneously. If it is sufficiently small, many of the neutrons will escape into the surroundings without causing further fission. The sample must exceed a critical mass before an explosion results. In an atomic bomb several pieces of fissionable material, all of which are below the critical mass, are held sufficiently far apart for no chain reaction to occur. When these fragments are suddenly brought together, an atomic explosion results immediately.
Bomb Construction
At Los Alamos, New Mexico, J. Robert Oppenheimer, and his research group were given the task of assembling and testing the fission bomb. Both types of fuel that were manufactured (U-235 and Pu-239) would be placed separately into two different bomb designs. The smaller of the two bombs would contain U-235 and was code-named "Little Boy." The larger bomb, which housed Pu-239, was named "Fat Man." Of the two, only the Pu-239 bomb would be tested before it was dropped in wartime.
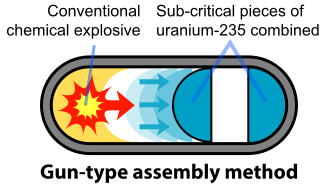
The basic design of the U-235 bomb is shown in the figure below. To prevent the spontaneous detonation of an atomic bomb, the fissile U-235 is kept in a subcritical configuration. It is then rapidly assembled into a supercritical mass using conventional explosives. Once the bomb has achieved this mass, any neutron introduced into it will be likely to initiate a chain reaction. The mechanism for "Little Boy" was a gun that fired one subcritical piece of U-235 into another to form a supercritical mass (Figure \(\PageIndex{3}\)). The pieces had to be assembled within a time less than the average time between appearances of spontaneous neutrons from either U-235 or cosmic radiation. A conventional explosive in an artillery barrel could fire the U-235 mass at speeds of a few millimeters per second, fast enough to prevent a fizzle caused by a spontaneous neutron setting off a premature chain reaction.
Originally, the gun-type mechanism was planned for both the U-235 and Pu-239 weapons. However, a problem arose with the Pu-239 bomb that required a different assembly mechanism because of the small amount of Pu-240 that is produced with the Pu-239 in the reactor. Pu-240 emits large numbers of neutrons spontaneously: 1,030 neutrons per gram per second compared with 0.0004 neutrons per gram per second for U-235. Even at a concentration of 1% Pu-240 in the fissile Pu-239, the required mass of Pu emits 52,000 neutrons per second or one neutron every 20 microseconds. Thus, it is very probable that a neutron from Pu-240 will initiate a premature chain reaction during the critical last 100 microseconds of the critical mass in a gun-type assembly. This problem was discovered in mid-1944, well after the start of the construction of the massive Hanford plutonium production facilities.
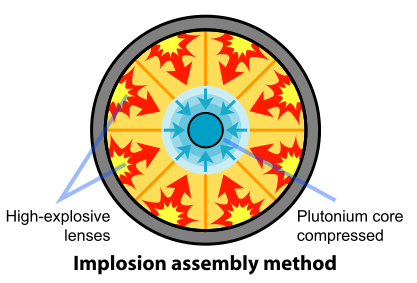
The removal of the Pu-240 was impractical. So the scientists and engineers looked for a faster method of assembling the plutonium. A mechanism based on implosion provided the solution to this problem. In this design, the fissile material is shaped into a single sphere with a mass slightly less than critical (Figure \(\PageIndex{4}\)). Layers of carefully shaped high explosives surround the sphere. When the explosives are detonated, the force of the shock wave compresses the fissile material into a smaller volume, forming a supercritical mass. This method of assembly is much faster than the gun-type mechanism and thus eliminates the problems resulting from spontaneous neutron emission of Pu-240. The spherical mass resulted in a pumpkin-shaped weapon called "Fat Man".
Watch the from the 23-minute mark through the 28-minute mark of the third installment of the History Channel's Manhattan Project and answer the questions below.
- What were the problems with the two different types of fuel that were to be used in the fission weapons?
- What was the configuration or set-up of the Pu-239 bomb?
- The combination of electromagnetic separation and gaseous diffusion enriched enough U-235 to produce how many bombs?
- Why was the U-235 bomb never tested?
- When was the construction of the U-235 completed?
- How much did Truman know about the nuclear bombs when FDR was alive?
- Who was selected to pilot the plane for the nuclear weapons?
- What island would serve as a base to house the nuclear bombs before delivering them to their targets?
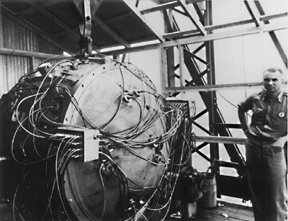
In July 1945, the United States had enough fissile material for one uranium and two plutonium weapons. The scientists and engineers felt confident that the gun-type assembly mechanism for Little Boy would function properly. Besides, they did not have the material for a test device. They were less confident about the implosion mechanism on the plutonium weapon and felt that a test was necessary. On July 16, 1945, the first nuclear device, known as "The Gadget", was placed on a 100-foot tower and successfully detonated in the Alamogordo Desert, 200 miles south of Los Alamos (Figure \(\PageIndex{5}\)).
As the war with Japan continued and a costly allied invasion loomed as a real possibility, President Truman approved the use of nuclear weapons against selected Japanese targets. The U.S. Army Air Force received orders to use these weapons anytime after August 3, 1945. On August 6, "Little Boy" was dropped on Hiroshima (Figure \(\PageIndex{6}\)).

Little Boy was a uranium weapon containing 141.4 pounds of fissionable material containing 82.7% U-235. Only about two pounds fissioned, releasing an energy equivalent to 15-16,000 tons of TNT. The immediate effects of the blast killed an estimated 70,000 people, and by the end of 1945 an additional 20,000 to 70,000 deaths occurred, many due to lack of adequate medical resources.
Three days later, "Fat Man" destroyed a large part of Nagasaki (Figure \(\PageIndex{7}\)). Fat Man contained 13.6 pounds of Pu-239, of which only 2 pounds underwent fission. The explosive yield was equivalent to about 22,000 tons of TNT. Fat man resulted in 35,000 immediate deaths. By the end of 1945, at least 70,000 people perished in this event.

Figure \(\PageIndex{7}\): Fat Man – One of many Pu-239 nuclear weapons constructed during WWII. Courtesy of the U.S. Department of Defense
Start the video below at the 28:00 mark and stop it at the 32-minute setting. Then, answer the questions below:
- When and where was the first nuclear bomb tested?
- Was the bomb placed on the ground?
- What were scientists afraid the first nuclear bomb would do?
- What did Edward Teller (father of the fusion bomb) apply to his skin before the bomb was ignited? Why did he do this?
- What did the bomb do to the tower and the surrounding sand?
- In the photograph, where did General Grove focus his attention?
Need More Practice?
In section 6E, work problem 6.
Contributors and Attributions
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).
- Muneeba Ali (Furman University)


