1.3: Pure and Applied Research
- Page ID
- 85133
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Distinguish between basic and applied chemistry
- Review the basics of the videos within in this section.
In science, we usually talk about two types of research: pure and applied. Pure research focuses on answering basic questions such as, "how do gases behave?" Applied research would be involved in the process of developing specific preparation for a gas in order for it to be produced and delivered efficiently and economically. This division sounds like it would be easy to make, but sometimes we cannot draw a clear line between what is "pure" and what is "applied".
Examples of "Pure" Research
A lot of "pure" research is of the "what is this?" or "how does it work?" variety. The early history of chemistry contains many examples. The ancient Greek philosophers debated the composition of matter (earth? air? fire? water? all of the above?). They did not intend to apply the knowledge gained from exploring the matter. Instead, they choose to focus on understanding the nature of matter.

Applied Research (Technology)
There is a great deal of "applied" research taking place today. In general, no new science principles are discovered, but existing knowledge is used to develop a new product. A good example of this type of research is the application of x-rays in medicine. In the later part of the 1800s, Wilhelm Röentgen discovered how to produce x-rays by using a cathode ray. He noted that this new ray could go through the body. Immediately, Röentgen believed that x-rays would be very helpful in diagnosing and determining disease and injury in the body.
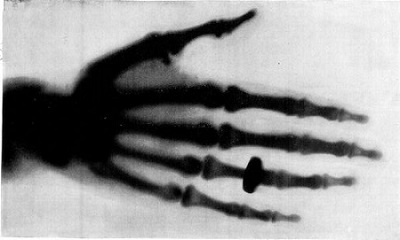
The Conversion of Basic Research to Applied Research
Scientists and engineers desire to understand how the universe works. Many times, this leads to unimaginable applications for their works. During the early 20th century, physicists and engineers performed experiments probing atoms with electricity and/or magnets. This research led to the identification of the three subatomic particles of the atom- electron, proton, and neutron. After determining these particles, physical chemists and physicists began experimenting on the central core of atoms (called nuclei). By bombarding a nucleus with other particles (beta, alpha, or neutron), they were able to transform one element into another.

Artificial transmutation can only occur in the nuclear lab. Large pieces of equipment are needed to change the nucleus. This discovery of altering the nucleus led to the production of new isotopes (atoms of the same element that differ in mass and neutron count). These new isotopes now are used in nuclear medicine (diagnosing and treating disease), nuclear weapons (producing fuel for bombs), sanitizing food, spices, and cosmetics, and nuclear energy.
Undergraduate Research at Furman University
All chemistry majors at our university are required to participate in undergraduate research. During the summertime, many of these students remain on campus to work in our laboratories. Students will join a group and research in one of the following areas: organic, inorganic, analytical, biochemistry, or physical chemistry. Some continue on their research in the calendar year. Upon graduation, all of our majors could enter industry immediately. Others will continue their chemical education by enrolling in Ph.D. programs. Lastly, many of our majors will continue on to become educators, patent attorneys, medical doctors, pharmacists, or physical therapists.
Listen to this 11-minute episode of the Shortwave Podcast. Answer the questions below about how Dr. Raychelle Burks distinguishes between good and bad science in the media.

- Did Dr. Burks always dream of becoming a scientist?
- How did Dr. Burks become interested in science?
- Before exploring academia, what kind of work did Dr. Burks do?
- Briefly explain the field of forensic science.
- According to Dr. Burks which shows get the science correct? Which shows are inaccurate?
- Provide some examples as to how the CSI shows have reported inaccurate science.
- How are machines over-glorified in crime based television shows?
- On the show, "Madam Secretary," what type of information did Dr. Burks provide to the show's, producers.
- List the two WMD chemicals that were mentioned by Dr. Burks. How should scientists be dressed when they encounter them?
- What type of chemist (recall the five types) is Dr. Burks?


