1.4: Classification and Properties of Matter
- Page ID
- 85134
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Use physical and chemical properties, including phase, to describe matter.
- Distinguish between physical and chemical changes.
- Identify a sample of matter as an element, a compound, or a mixture (homogeneous/heterogeneous).
- Distinguish between organic and inorganic chemicals.
- Label all atoms within an organic or inorganic compound.
- Recognize physical methods used to separate mixtures.
Physical and Chemical Properties
The properties that chemists use to describe matter fall into two general categories. Physical properties are characteristics that describe matter. They include characteristics such as size, shape, color, and mass. Many of these properties can be quantitative in nature. For example, quantitative physical properties of water would be the boiling point (100 °C / 212 °F) and melting point (0°C / 32 °F).

Figure \(\PageIndex{1}\): Physical Properties. (Copyright;https://www.slideshare.net/cfoltz/ph...cal-properties)
Chemical properties are characteristics that describe how matter changes its chemical structure or composition. An example of a chemical property is flammability—a material’s ability to burn—because burning (also known as combustion) changes the chemical composition of a material. Oxidation, rusting, decomposition, and inertness are chemical properties as well. Click on this video and record the physical and chemical properties of the element sodium.
Elements and Compounds
Any sample of matter that has the same physical and chemical properties throughout the sample is called a substance. There are two types of substances. A substance that cannot be broken down into chemically simpler components is an element. Aluminum, which is used in soda cans, is an element. A substance that can be broken down into chemically simpler components (because it has more than one element) is a compound (Figure \(\PageIndex{1}\)). Water is a compound composed of the elements hydrogen and oxygen. Today, there are 118 elements in the known universe. In contrast, scientists have identified tens of millions of different compounds to date.
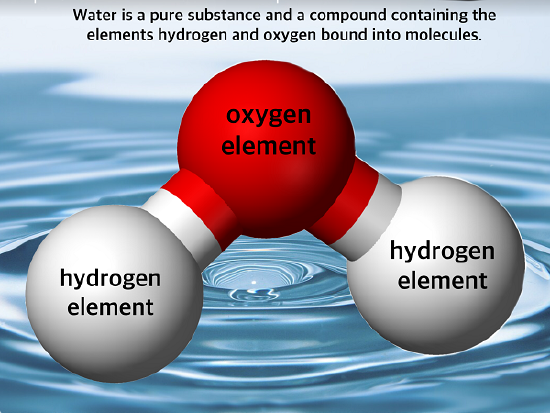
Sometimes the word pure is added to a substance, but this is not absolutely necessary. By definition, any single substance is pure.
The smallest part of an element that maintains the identity of that element is called an atom. Atoms are extremely tiny; to make a line 1 inch long, you would need 217 million iron atoms. The smallest part of a compound that maintains the identity of that compound is called a molecule. Molecules are composed of atoms that are attached together and behave as a unit. Scientists usually work with millions and millions of atoms and molecules at a time. When a scientist is working with large numbers of atoms or molecules at a time, the scientist is studying the macroscopic view of the universe.
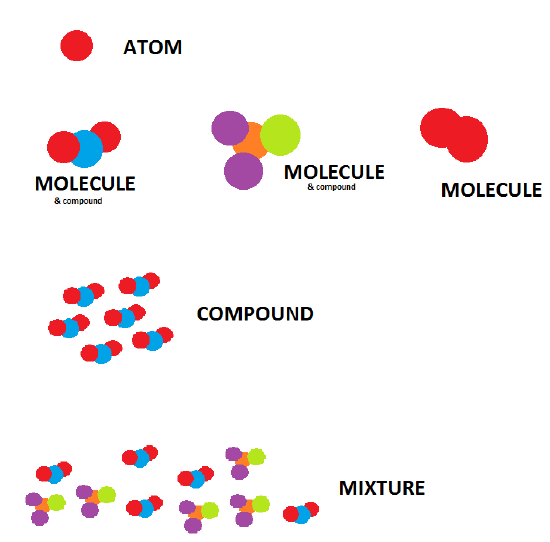
Figure \(\PageIndex{4}\): Appreciating elements, molecules and compounds.
Inorganic and Organic Compounds
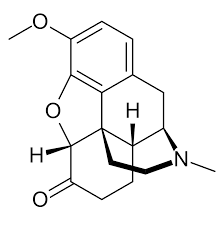
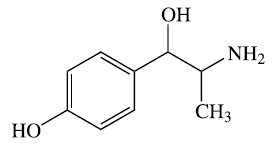
Compounds can be further classified as being inorganic or organic. Organic compounds contain carbon, hydrogen, and usually another element from the right side of the periodic table. Be careful when thinking about organic chemicals. In the real world, the term "organic" is typically used to describe products that have natural ingredients. In the world of science, the word "organic" is used to denote compounds that have carbon and hydrogen connections. Look at some of the structures below to get an appreciation of organic chemical structures.
The two molecules shown below are heavily used in the pharmaceutical industry. The compound on the left is known as hydrocodone. This is an opioid painkiller that is extremely addictive. It is regularly dispensed for surgical and chronic pain. The compound on the right is Adderall. This medication is used to treat ADHD and narcolepsy. Both of these medications are controlled by the DEA (Drug Enforcement Agency) and are illegal to possess unless you have a valid prescription.
Mixtures
A material composed of two or more substances is a mixture. In a mixture, the individual substances maintain their chemical identities. Many mixtures are obvious combinations of two or more substances, such as a mixture of sand and water. Such mixtures are called heterogeneous mixtures. In some mixtures, the components are so intimately combined that they act like a single substance (even though they are not). Mixtures with a consistent composition throughout are called homogeneous mixtures (or solutions). Sugar dissolved in water is an example of a solution. A metal alloy, such as steel, is an example of a solid solution. Air, a mixture of mainly nitrogen and oxygen, is a gaseous solution.
How would a chemist categorize each example of matter?
- saltwater
- soil
- water
- oxygen
Solution
- Saltwater acts as if it were a single substance even though it contains two substances—salt and water. Saltwater is a homogeneous mixture, or a solution.
- Soil is composed of small pieces of a variety of materials, so it is a heterogeneous mixture.
- Water is a substance; more specifically, because water is composed of hydrogen and oxygen, it is a compound.
- Oxygen, a substance, is an element.
How would a chemist categorize each example of matter?
- coffee
- hydrogen
- an egg
- Answer a
-
Coffee could be a heterogeneous or homogeneous mixture. If insoluble parts are able to be identified, then it would be classified as being heterogeneous. If not, then coffee would be a homogeneous mixture or a solution.
- Answer b
-
Hydrogen is an element. It can be located on the periodic table.
- Answer c
-
An egg (assume raw) would be a heterogeneous mixture. The yolk and egg white are two different substances that can be easily seen.
Phases
Another way to classify matter is to describe it as a solid, a liquid, or a gas, which was done in the examples of solutions. These three descriptions, each implying that the matter has certain physical properties, represent the three phases of matter. A solid has a definite shape and a definite volume. Liquids ordinarily have a definite volume but not a definite shape; they take the shape of their containers. Gases have neither a definite shape nor a definite volume, and they expand to fill their containers. We encounter matter in each phase every day; in fact, we regularly encounter water in all three phases: ice (solid), water (liquid), and steam (gas).
We know from our experience with water that substances can change from one phase to another if the conditions are right. Typically, varying the temperature of a substance (and, less commonly, the pressure exerted on it) can cause a phase change, a physical process in which a substance goes from one phase to another (Figure \(\PageIndex{3}\)). Phase changes have particular names depending on what phases are involved, as summarized in Table \(\PageIndex{1}\).
| Change | Name |
|---|---|
| solid to liquid | melting, fusion |
| solid to gas | sublimation |
| liquid to gas | boiling, evaporation |
| liquid to solid | solidification, freezing |
| gas to liquid | condensation |
| gas to solid | deposition |

- Classify each as being element, compound, heterogeneous mixture, or homogeneous mixture (solution): air at Furman University, Furman Lake water, copper wire, and Furman tap water.
- What is the difference between a heterogeneous mixture and a homogeneous mixture? Give an example of each.
- Which is/are physical change(s): burning of fuel, digestion of food, salt dissolving in water, copper conducting electricity, and water boiling.
- Answer a
-
Hopefully, Furman air is a homogenous mixture (solution). If there is no burning or dust build-up in the area, this is safe to assume. Furman Lake is definitely a heterogeneous mixture (in some areas it can get a bit swampy). Copper wire is an element. There are very few elements in nature. Lastly, Furman tap water is usually a homogeneous mixture. Natural and man-made chemicals are in our tap water (trust me, you want the chlorine). Our toilets do use greywater which can look heterogeneous (ok for sewer but not to drink).
- Answer b
-
A heterogeneous mixture is obviously a mixture, such as dirt; a homogeneous mixture behaves like a single substance, such as saltwater.
- Answer c
-
The physical changes are salt dissolving water, copper conducting, and water boiling. If composition is not changed, then this is a physical change. If there is a method to physically separate the components (like boiling, evaporation, distillation, magnetism, or filtration), then a physical change has occurred.
Contributors and Attributions
Anonymous
- Hayden Cox (Furman University)


