16.2: Amino Acid Catabolism
- Page ID
- 337695
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Breakdown of glutamine by glutaminase is a source of ammonium ion in the cell. The other product is glutamate. Glutamate, of course, can be converted by a transamination reaction to alpha-ketoglutarate, which can be oxidized in the citric acid cycle.
- Asparagine can similarly be broken to ammonium and aspartate by asparaginase and aspartate can be converted by transamination to oxaloacetate for oxidation in the citric acid cycle.
- Alanine is converted to pyruvate in a transamination reaction, making it glucogenic.
- Arginine is hydrolzyed in the urea cycle to yield urea and ornithine.
- Proline is catabolized to glutamate in a reversal of its synthesis pathway.
- Serine donates a carbon to form a folate and the other product of the reaction is glycine, which is itself oxidized to carbon dioxide and ammonia. Glycine can also be converted back to serine, which can also be converted back to 3-phosphoglycerate or pyruvate.
- Threonine can be broken down in three pathways, though only two are relevant for humans. One pathway leads to acetyl-CoA and glycine. The other leads to pyruvate.
- Cysteine can be broken down in several ways. The simplest occurs in the liver, where a desulfurase can act on it to yield hydrogen sulfide and pyruvate.
- Methionine can be converted to cysteine for further metabolism. It can be converted to succinyl-CoA for oxidation in the citric acid cycle. It can also be converted to S-Adenosyl-Methionine (SAM), a carbon donor.
- Isoleucine and valine can also be converted to succinyl-CoA after conversion first to propionyl-CoA. Since conversion of propionyl-CoA to succinyl-CoA requires vitamin \(\text{B}_{12}\), catabolism of these amino acids also requires the vitamin.
- Phenylalanine is converted during catabolism to tyrosine, which is degraded ultimately to fumarate and acetoacetate. Thus, both of these amino acids are glucogenic and ketogenic. Tyrosine can also be converted to dopamine, norepinephrine, and epinephrine.
- Leucine and lysine can be catabolized to acetoacetate and acetyl-CoA. Lysine is also an important precursor of carnitine.
- Histidine can be catabolized by bacteria in intestines to histamine, which causes construction or dilation of various blood vessels when in excess.
- Tryptophan’s catabolism is complex, but can proceed through alanine, acetoacetate and acetyl-CoA
In summary, the following are metabolized to pyruvate – alanine, cysteine, glycine, serine, and threonine
- Oxaloacetate is produced from aspartate and asparagine
- Succinyl-CoA is produced from isoleucine, valine, and methionine
- Alpha-ketoglutarate is produced from arginine, glutamate, glutamine, histidine and proline.
- Phenylalanine and tyrosine are broken down to fumarate and acetoacetate
- Leucine and lysine yield acetoacetate and acetyl-CoA.
- Tryptophan leads to alanine, acetoacetate and acetyl-CoA.
Last, amino acids, besides being incorporated into proteins, serve as precursors of important compounds, including serotonin (from tryptophan), porphyrin heme (from glycine), nitric oxide (from arginine), and nucleotides (from aspartate, glycine, and glutamine).
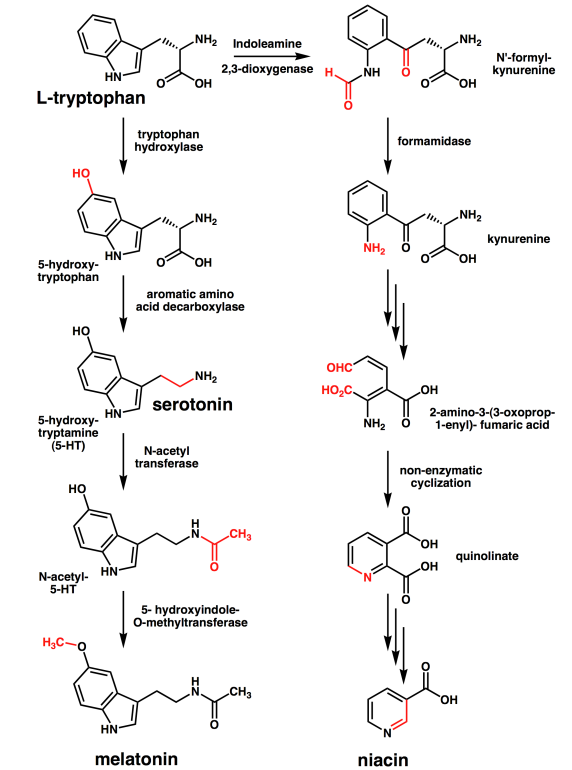 Figure 7.8.1: Conversion of L-Tryptophan into Serotonin, Melatonin, and Niacin
Figure 7.8.1: Conversion of L-Tryptophan into Serotonin, Melatonin, and Niacin


