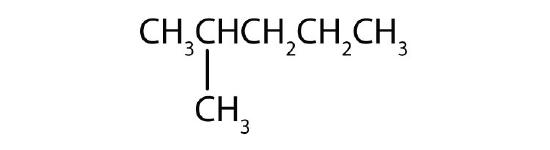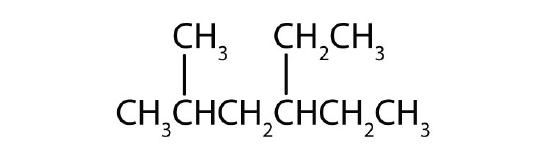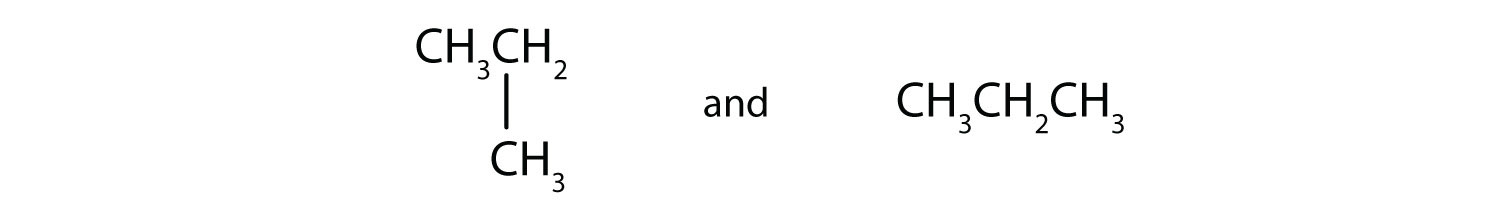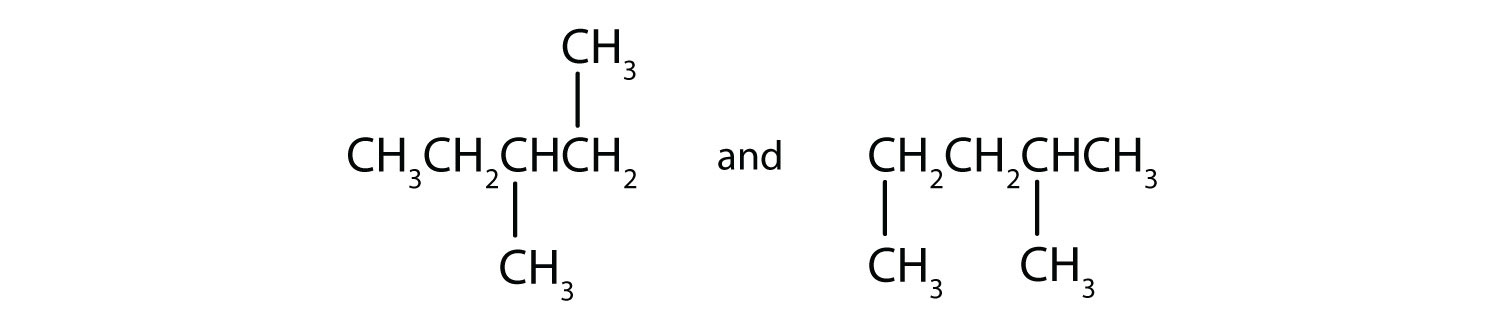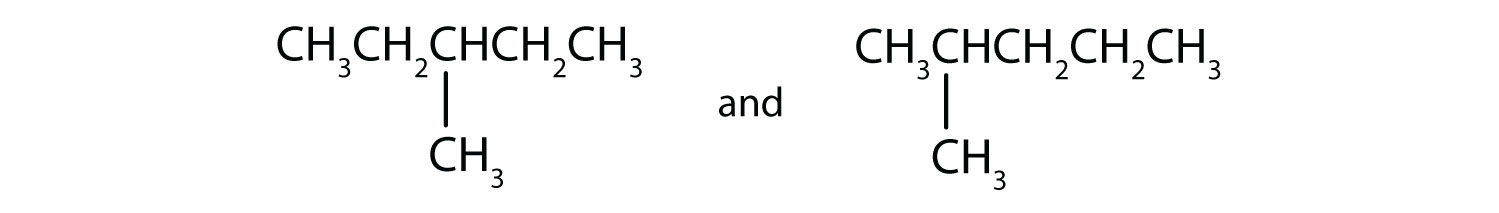1.13: Organic Chemistry- Alkanes and Halogenated Hydrocarbons (Exercises)
- Page ID
- 337788
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
12.1: Organic Chemistry
Concept Review Exercises
-
Classify each compound as organic or inorganic.
- C3H8O
- CaCl2
- Cr(NH3)3Cl3
- C30H48O3N
-
Which compound is likely organic and which is likely inorganic?
- a flammable compound that boils at 80°C and is insoluble in water
- a compound that does not burn, melts at 630°C, and is soluble in water
-
Classify each compound as organic or inorganic.
- C6H10
- CoCl2
- C12H22O11
-
Classify each compound as organic or inorganic.
- CH3NH2
- NaNH2
- Cu(NH3)6Cl2
-
Which member of each pair has a higher melting point?
- CH3OH and NaOH
- CH3Cl and KCl
-
Which member of each pair has a higher melting point?
- C2H6 and CoCl2
- CH4 and LiH
Answers
-
- organic
- inorganic
- inorganic
- organic
-
- organic
- inorganic
-
a. organic b. inorganic c. organic
- NaOH
- KCl
Concept Review Exercises
-
In alkanes, can there be a two-carbon branch off the second carbon atom of a four-carbon chain? Explain.
-
A student is asked to write structural formulas for two different hydrocarbons having the molecular formula C5H12. She writes one formula with all five carbon atoms in a horizontal line and the other with four carbon atoms in a line, with a CH3 group extending down from the first attached to the third carbon atom. Do these structural formulas represent different molecular formulas? Explain why or why not.
Answers
-
No; the branch would make the longest continuous chain of five carbon atoms.
-
No; both are five-carbon continuous chains.
Key Takeaway
- Alkanes with four or more carbon atoms can exist in isomeric forms.
Exercises
-
Briefly identify the important distinctions between a straight-chain alkane and a branched-chain alkane.
-
How are butane and isobutane related? How do they differ?
-
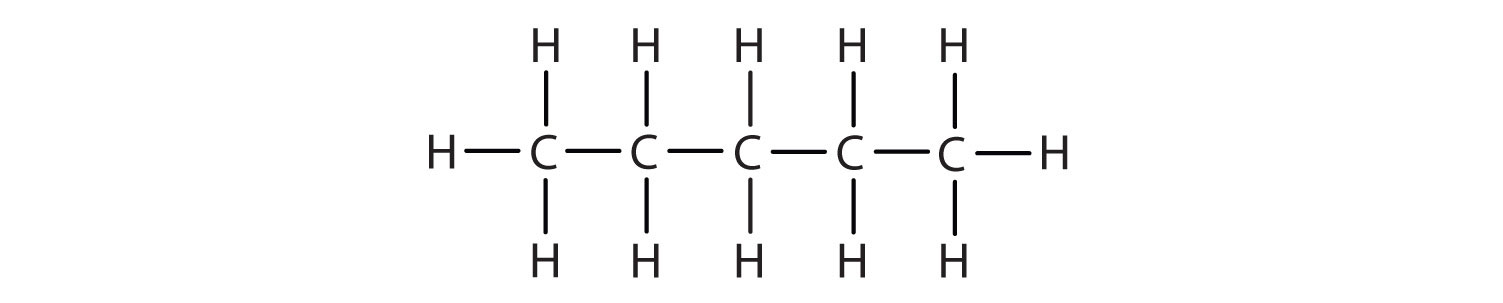
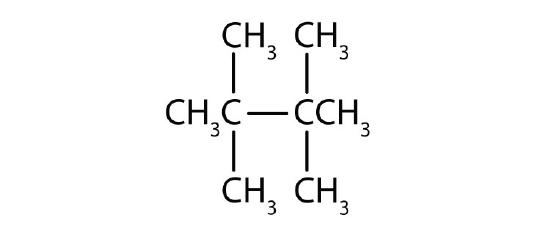
Name each compound.
-
-
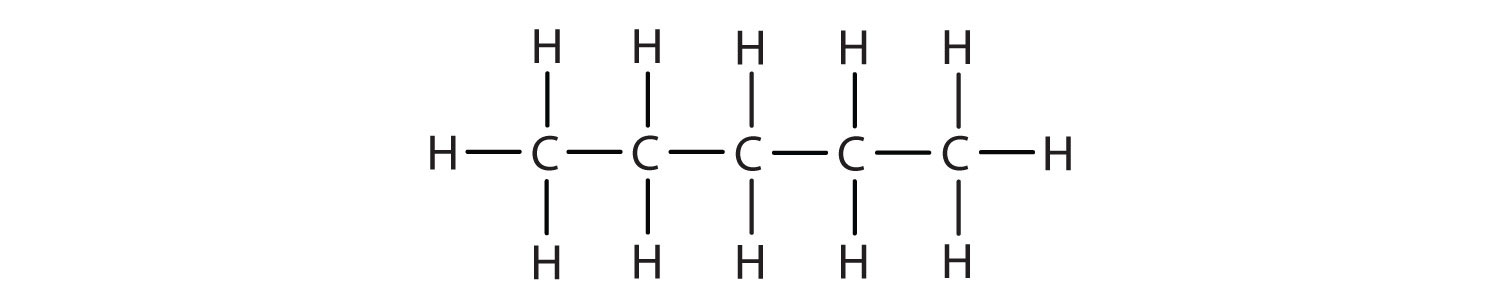
Write the structural formula for each compound.
- hexane
- octane
-
Indicate whether the structures in each set represent the same compound or isomers.
-
CH3CH2CH2CH3 and

-
CH3CH2CH2CH2CH3 and
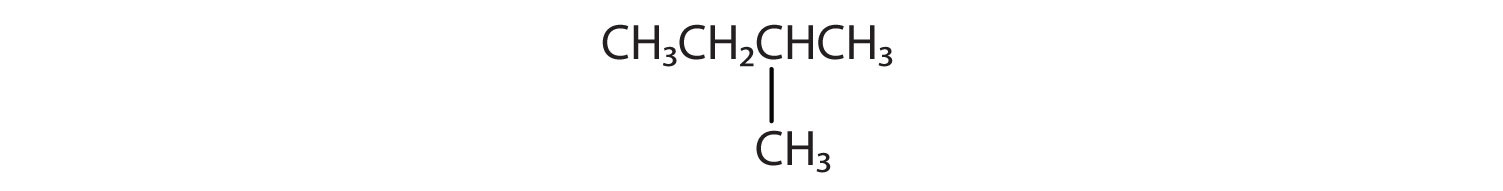
-
Answers
-
Straight-chain alkanes and branched-chain alkanes have different properties as well as different structures.
-
- pentane
- heptane
-
- not isomers, same compound
- yes, isomers
Exercises
-
Write the condensed structural formula for each structural formula.
-
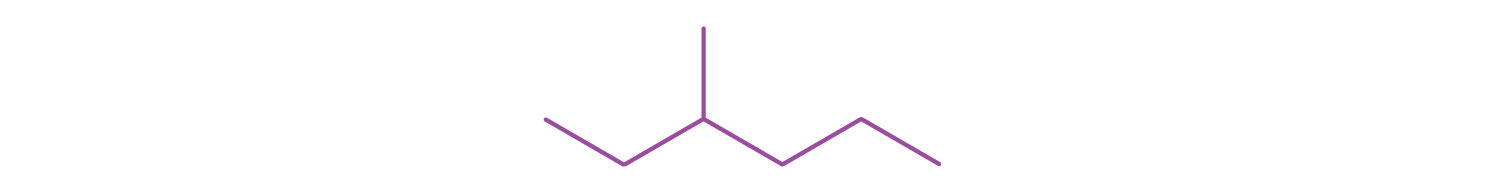
-
A condensed structural formula for isohexane can be written as
 . Draw the line-angle formula for isohexane.
. Draw the line-angle formula for isohexane. -
Draw a line-angle formula for the compound

-
Give the condensed structural formula for the compound represented by this line-angle formula:
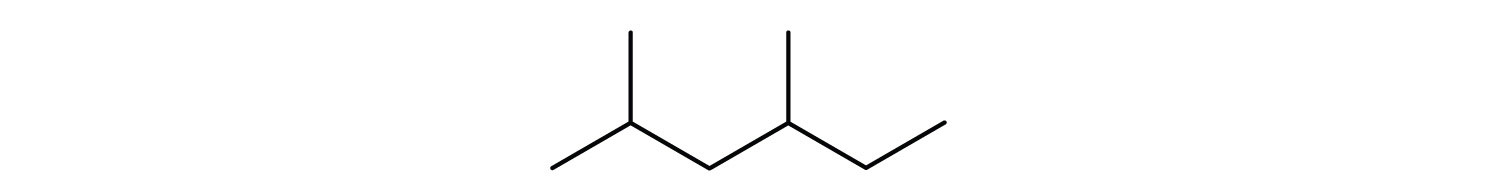
Answers
-
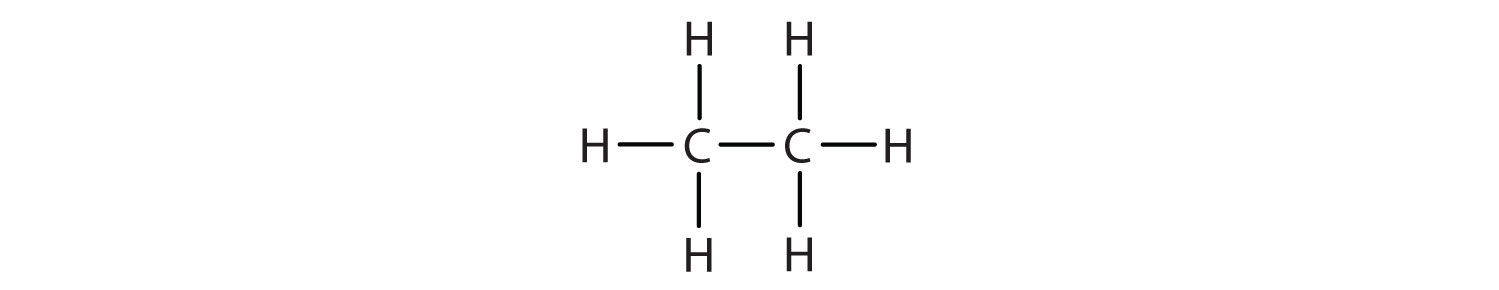
- CH3CH3
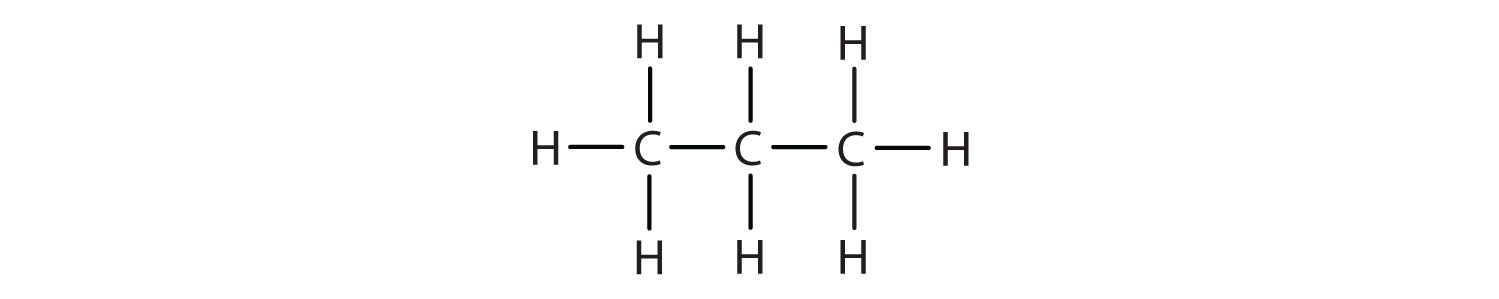
- CH3CH2CH3
- CH3CH2CH2CH2CH3
Concept Review Exercises
- What is a CH3 group called when it is attached to a chain of carbon atoms—a substituent or a functional group?
- Which type of name uses numbers to locate substituents—common names or IUPAC names?
Answers
-
substituent
-
IUPAC names
Exercises
-
Briefly identify the important distinctions between an alkane and an alkyl group.
-
How many carbon atoms are present in each molecule?
- 2-methylbutane
- 3-ethylpentane
-
How many carbon atoms are present in each molecule?
- 2,3-dimethylbutane
- 3-ethyl-2-methylheptane
-
Draw the condensed structure for each compound.
- 3-methylpentane
- 2,2,5-trimethylhexane
- 4-ethyl-3-methyloctane
-
Draw the condensed structure for each compound.
- 2-methylpentane
- 4-ethyl-2-methylhexane
- 2,2,3,3-tetramethylbutane
-
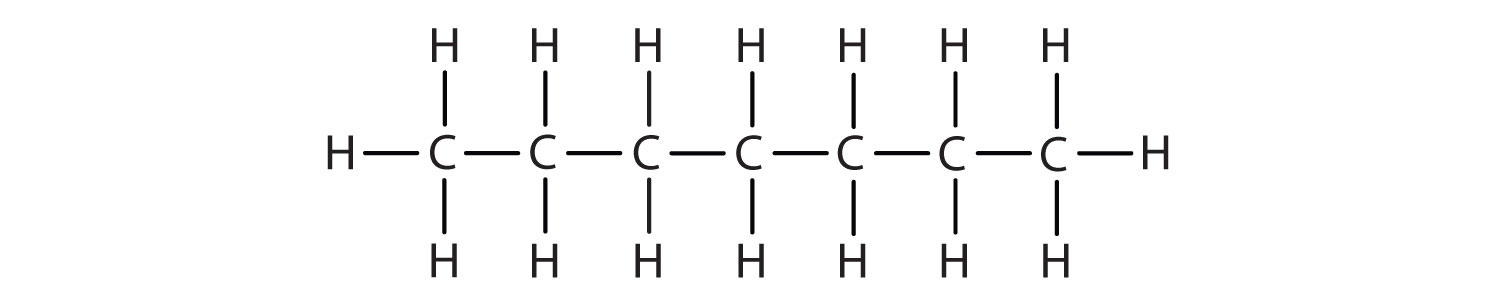
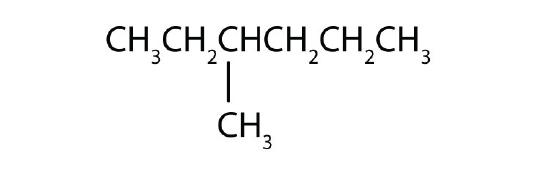
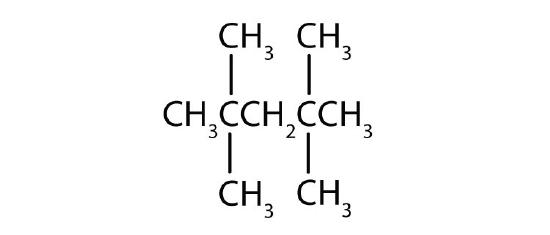
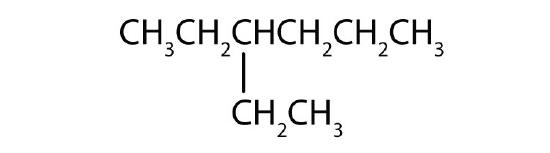
Name each compound according to the IUPAC system.
-
-
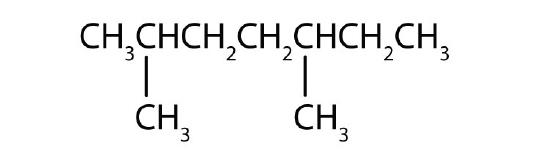
Name each compound according to the IUPAC system.
-
-
What is a substituent? How is the location of a substituent indicated in the IUPAC system?
-
Briefly identify the important distinctions between a common name and an IUPAC name.
Answers
-
An alkane is a molecule; an alkyl group is not an independent molecule but rather a part of a molecule that we consider as a unit.
-
- 6
- 10
-
- 2,2,4,4-tetramethylpentane
- 3-ethylhexane
-
Common names are widely used but not very systematic; IUPAC names identify a parent compound and name other groups as substituents.
Additional Exercises
-
You find an unlabeled jar containing a solid that melts at 48°C. It ignites readily and burns readily. The substance is insoluble in water and floats on the surface. Is the substance likely to be organic or inorganic?
-
Give the molecular formulas for methylcyclopentane, 2-methylpentane, and cyclohexane. Which are isomers?
-
What is wrong with each name? (Hint: first write the structure as if it were correct.) Give the correct name for each compound.
- 2-dimethylpropane
- 2,3,3-trimethylbutane
- 2,4-diethylpentane
- 3,4-dimethyl-5-propylhexane
-
What is the danger in swallowing a liquid alkane?
-
Distinguish between lighter and heavier liquid alkanes in terms of their effects on the skin.
-
Following is the line formula for an alkane. Draw its condensed structure and give its name.
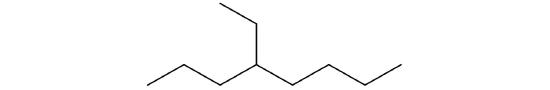
-
Write equations for the complete combustion of each compound.
- propane (a bottled gas fuel)
- octane (a typical hydrocarbon in gasoline).
-
Skip
-
Draw the structures for the five isomeric hexanes (C6H14). Name each by the IUPAC system.
-
Indicate whether the structures in each set represent the same compound or isomers.
-
-
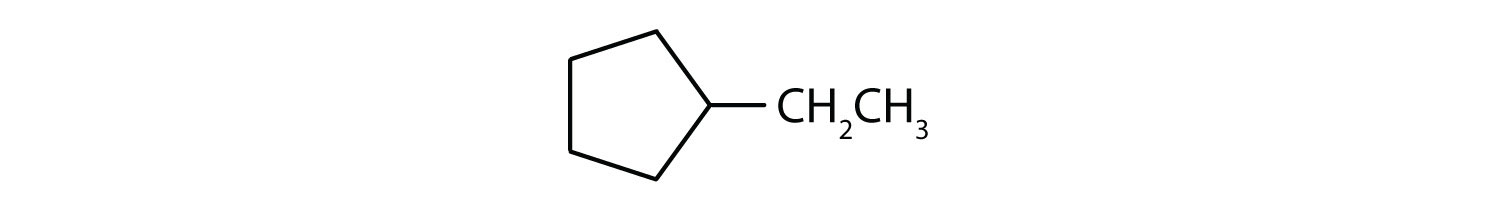
Consider the line-angle formulas shown here and answer the questions.
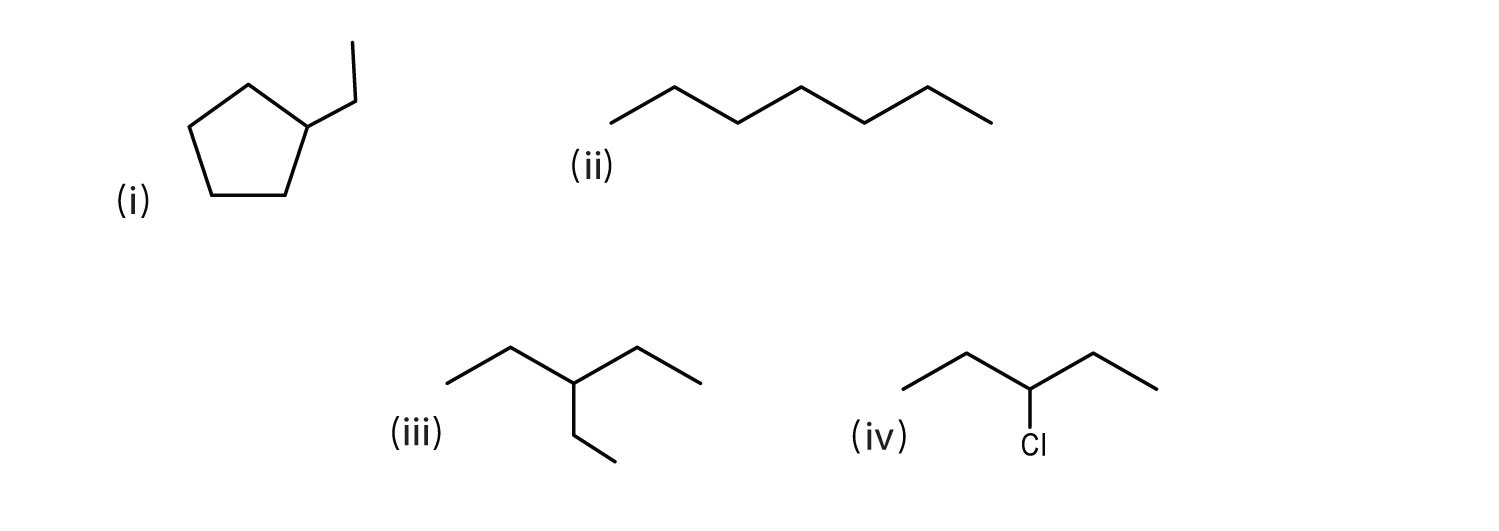
- Which pair of formulas represents isomers? Draw each structure as condensed.
- Which formula represents an alkyl halide? Name the compound and write its condensed structural formula.
- Which formula represents a cyclic alkane? Name the compound and draw its structure.
- What is the molecular formula of the compound represented by (i)?
Answers
-
organic
-
- Two numbers are needed to indicate two substituents; 2,2-dimethylpropane.
- The lowest possible numbers were not used; 2,2,3-trimethylbutane.
- An ethyl substituent is not possible on the second carbon atom; 3,5-dimethylheptane.
- A propyl substituent is not possible on the fifth carbon atom; 3,4,5-trimethyloctane.
-
Lighter alkanes wash away protective skin oils; heavier alkanes form a protective layer.
-
- C3H8 + 5O2 → 3CO2 + 4H2O
- 2C8H18 + 25O2 → 16CO2 + 18H2O
-
CH3CH2CH2CH2CH2CH3; hexane
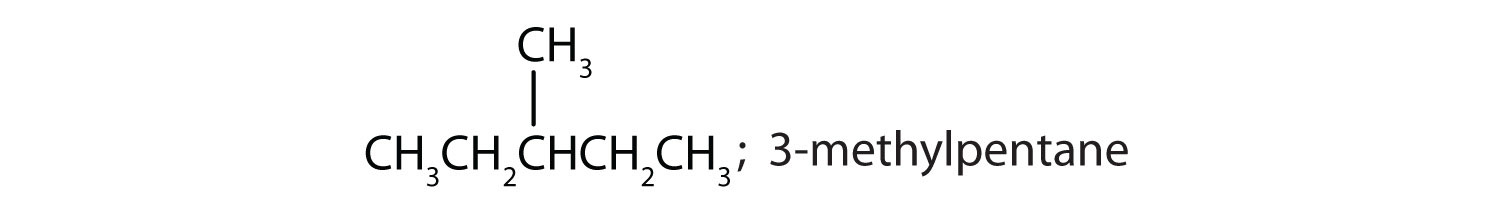
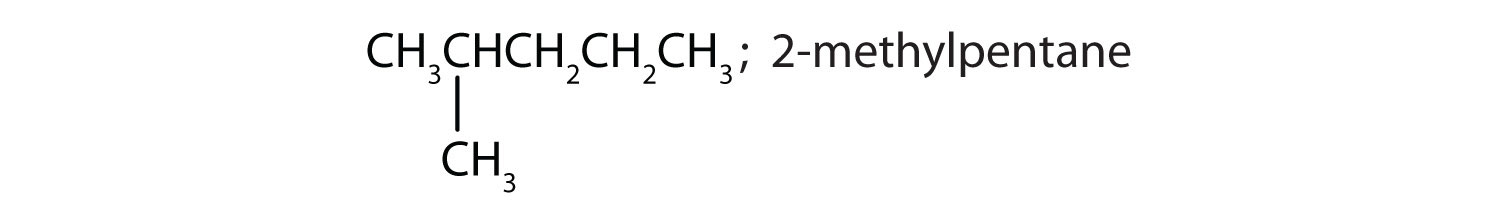
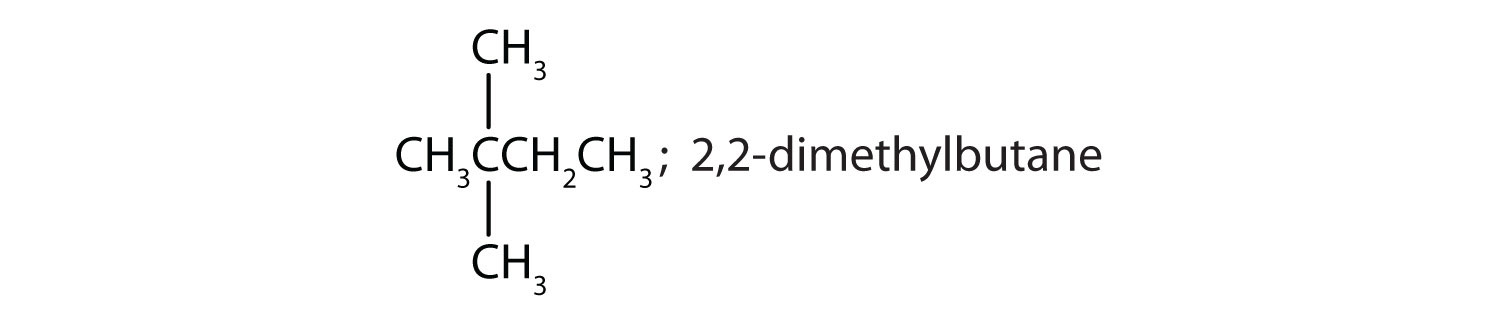
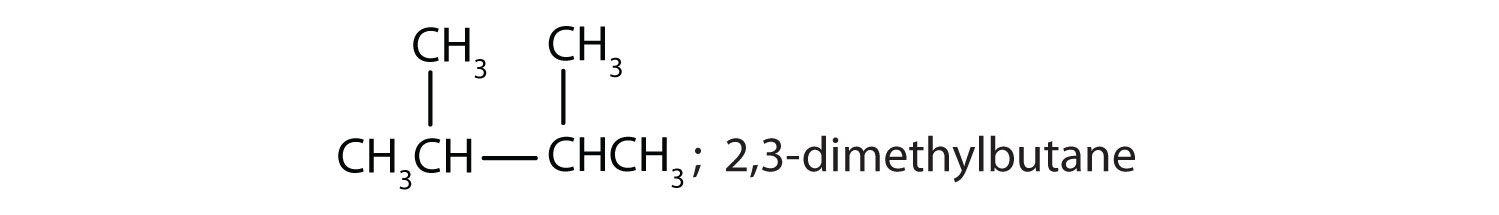
-
-
ii and iii; CH3CH2CH2CH2CH2CH2CH3 and
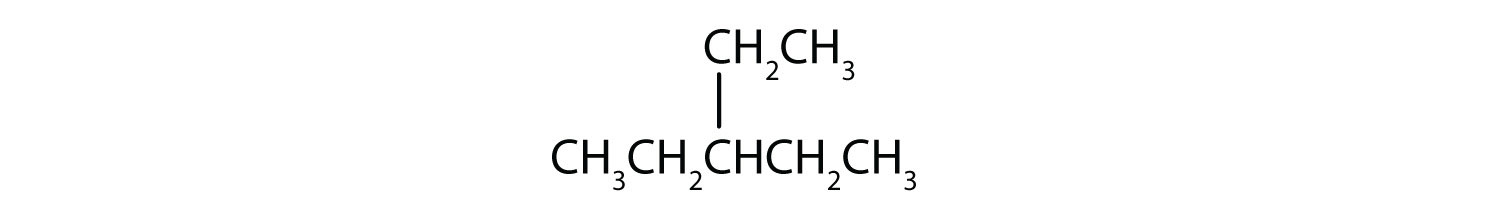
- iv; 3-chloropentane;
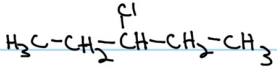
-
i; ethylcyclopentane;
- C7H14
-