_xx_ delete-test
- Page ID
- 449333
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Describe how electrons are grouped within atoms.
- Write electron configurations for atoms.
- Connect the electron configuration of atoms to the energy levels of the electrons in the atom.
Previously we discussed the concept of electron shells and subshells. It is the arrangement of electrons into shells and subshells that most concerns us here, so we will focus on that.
General Rules of Electron Configuration
There are a set of general rules that are used to figure out the electron configuration of an atomic species: Aufbau Principle, Hund's Rule and the Pauli-Exclusion Principle. Before continuing, it's important to understand that each orbital can be occupied by two electrons.
- Rule 1 (Aufbau Principle): Electrons occupy the lowest-energy orbitals possible, starting with 1s and continuing in the order dictated by quantum mechanics
- Rule 2 (Hund's Rule): Electrons occupy degenerate orbitals (i.e. same \(n\) and \(\ell\) quantum numbers), they must first occupy the empty orbitals before double occupying them. Furthermore, the most stable configuration results when the spins are parallel (i.e. all same \(m_s\) quantum numbers).
- Rule 3 (Pauli-Exclusion Principle): Each electron can be described with a unique set of four quantum numbers. Therefore, if two electrons occupy the same orbital, they have different spin - spin up or spin down.
Electron Configurations
Electron configurations are are shorthand descriptions of the arrangements of electrons in atoms. An example electron configuration with its general structure is shown in Figure \(\PageIndex{1}\). In electron configurations, we use numbers to indicate which shell an electron is in.

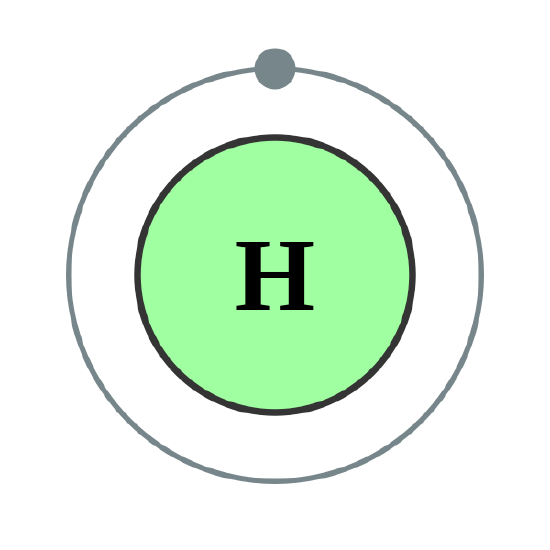
As shown in Table \(\PageIndex{1}\), the first shell, closest to the nucleus and with the lowest-energy electrons, is shell 1. This first shell has only one subshell, which is labeled 1s and can hold a maximum of 2 electrons. We combine the shell and subshell labels when referring to the organization of electrons about a nucleus and use a superscript to indicate how many electrons are in a subshell. Thus, because a hydrogen atom has its single electron in the s subshell of the first shell, we use 1s1 to describe the electronic structure of hydrogen.
| Shell | Number of Subshells | Names of Subshells |
|---|---|---|
| 1 | 1 | 1s |
| 2 | 2 | 2s and 2p |
| 3 | 3 | 3s, 3p and 3d |
| 4 | 4 | 4s, 4p, 4d and 4f |
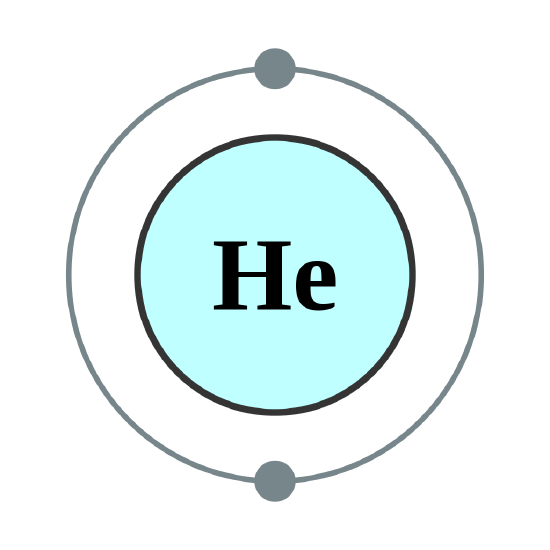
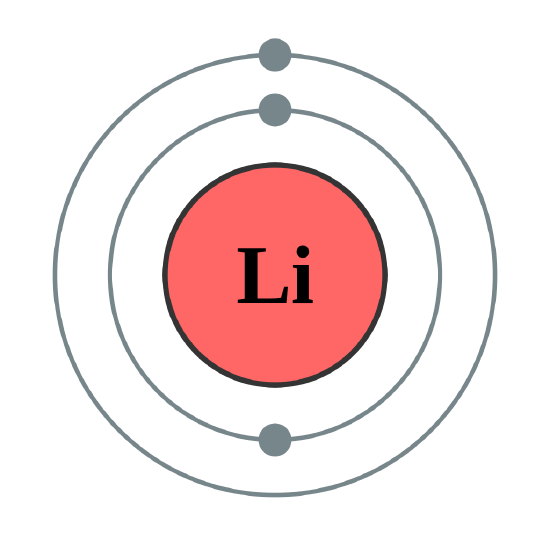
Helium atoms have 2 electrons. Both electrons fit into the 1s subshell because s subshells can hold up to 2 electrons; therefore, the electron configuration for helium atoms is 1s2 (spoken as “one-ess-two”). Different subshells hold a different maximum number of electrons. Any s subshell can hold up to 2 electrons; p, 6; d, 10; and f, 14 (Table \(\PageIndex{2}\)). Hence, the 1s subshell cannot hold 3 electrons (because an s subshell can hold a maximum of 2 electrons), so the electron configuration for a lithium atom cannot be 1s3 (Figure \(\PageIndex{2}\)). Two of the lithium electrons can fit into the 1s subshell, but the third electron must go into the second shell. The second shell has two subshells, s and p, which fill with electrons in that order. The 2s subshell holds a maximum of 2 electrons, and the 2p subshell holds a maximum of 6 electrons. Because lithium’s final electron goes into the 2s subshell, we write the electron configuration of a lithium atom as 1s22s1. The shell diagram for a lithium atom (Figure \(\PageIndex{1}\)). The shell closest to the nucleus (first shell) has 2 dots representing the 2 electrons in 1s, while the outermost shell (2s) has 1 electron.
| Subshell | Maximum Number of Electrons |
|---|---|
| s | 2 |
| p | 6 |
| d | 10 |
| f | 14 |
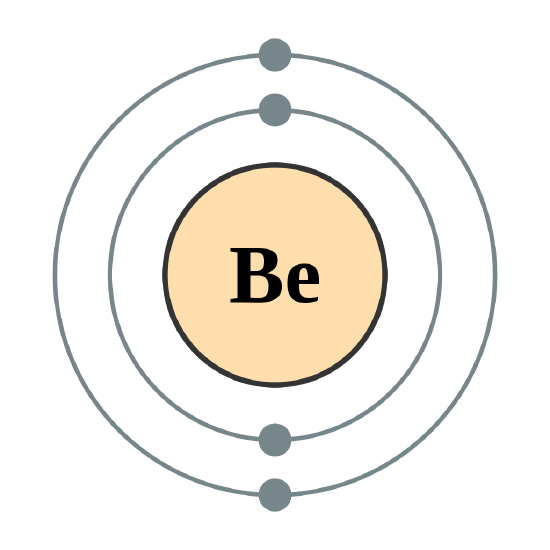
The next largest atom, beryllium, has 4 electrons, so its electron configuration is 1s22s2. Now that the 2s subshell is filled, electrons in larger atoms start filling the 2p subshell. With neon, the 2p subshell is completely filled. Because the second shell has only two subshells, atoms with more electrons now must begin the third shell. The third shell has three subshells, labeled s, p, and d. The d subshell can hold a maximum of 10 electrons. The first two subshells of the third shell are filled in order—for example, the electron configuration of aluminum, with 13 electrons, is 1s22s22p63s23p1. However, a curious thing happens after the 3p subshell is filled: the 4s subshell begins to fill before the 3d subshell does. In fact, the exact ordering of subshells becomes more complicated at this point (after argon, with its 18 electrons), so we will not consider the electron configurations of larger atoms. A fourth subshell, the f subshell, is needed to complete the electron configurations for all elements. An f subshell can hold up to 14 electrons.
| Z | Element | Outer most Shell | Configuration | Noble Gas Configuration |
|---|---|---|---|---|
| 1 | H | 1 | 1s 1 | 1s 1 |
| 2 | He | 1 | 1s 2 | 1s 2 |
| 3 | Li | 2 | 1s 2 2s 1 | [He] 2s 1 |
| 4 | Be | 2 | 1s 2 2s 2 | [He] 2s 2 |
| 5 | B | 2 | 1s 2 2s 2 2p1 | [He] 2s 2 2p1 |
| 6 | C | 2 | 1s 2 2s 2 2p2 | [He] 2s 2 2p2 |
| 7 | N | 2 | 1s 2 2s 2 2p3 | [He] 2s 2 2p3 |
| 8 | O | 2 | 1s 2 2s 2 2p4 | [He] 2s 2 2p4 |
| 9 | F | 2 | 1s 2 2s 2 2p5 | [He] 2s 2 2p5 |
| 10 | Ne | 2 | 1s 2 2s 2 2p6 | [He] 2s 2 2p6 |
| 11 | Na | 3 | 1s 2 2s 2 2p6 3s 1 | [Ne] 3s 1 |
| 12 | Mg | 3 | 1s 2 2s 2 2p6 3s 2 | [Ne] 3s 2 |
| 13 | Al | 3 | 1s 2 2s 2 2p6 3s 2 3p1 | [Ne] 3s 2 3p1 |
| 14 | Si | 3 | 1s 2 2s 2 2p6 3s 2 3p2 | [Ne]3s 2 3p2 |
| 15 | P | 3 | 1s 2 2s 2 2p6 3s 2 3p3 | [Ne] 3s 2 3p3 |
| 16 | S | 3 | 1s 2 2s 2 2p6 3s 2 3p4 | [Ne] 3s 2 3p4 |
| 17 | Cl | 3 | 1s 2 2s 2 2p6 3s 2 3p5 | [Ne] 3s 2 3p5 |
| 18 | Ar | 3 | 1s 2 2s 2 2p6 3s 2 3p6 | [Ne] 3s 2 3p6 |
| 19 | K | 4 | 1s 2 2s 2 2p6 3s 2 3p6 4s 1 | [Ar] 4s 1 |
| 20 | Ca | 4 | 1s 2 2s 2 2p6 3s 2 3p6 4s 2 | [Ar] 4s 2 |
Electron filling always starts with 1s, the subshell closest to the nucleus. Next is 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, etc., shown in the electron shell filling order diagram in Figure \(\PageIndex{3}\). Follow each arrow in order from top to bottom. The subshells you reach along each arrow give the ordering of filling of subshells in larger atoms.
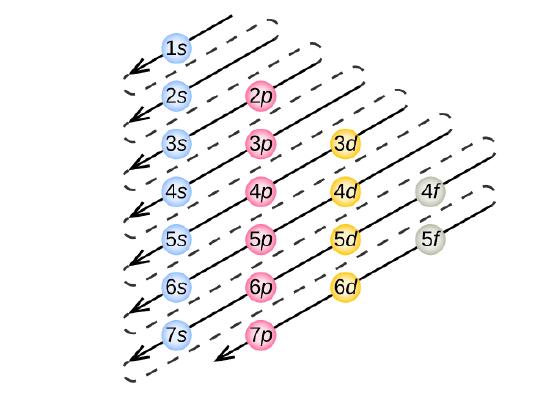
Noble Gas Configuration
The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\) (Table \(\PageIndex{3}\)). The first ten electrons of the sodium atom are the inner-shell electrons and the configuration of just those ten electrons is exactly the same as the configuration of the element neon \(\left( Z=10 \right)\). This provides the basis for a shorthand notation for electron configurations called the noble gas configuration, which atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the substitution of \(\left[ \ce{Ne} \right]\) for the \(1s^2 2s^2 2p^6\) part of the configuration. Sodium's noble gas configuration becomes \(\left[ \ce{Ne} \right] 3s^1\). Table \(\PageIndex{1}\) shows the noble gas configurations of the third period elements.
Electron Configurations and Orbital Diagrams
We construct the periodic table by following the aufbau principle (from German, meaning “building up”). First we determine the number of electrons in the atom; then we add electrons one at a time to the lowest-energy orbital available without violating the Pauli principle. We use the orbital energy diagram of Figure \(\PageIndex{1}\), recognizing that each orbital can hold two electrons, one with spin up ↑, corresponding to ms = +½, which is arbitrarily written first, and one with spin down ↓, corresponding to ms = −½. A filled orbital is indicated by ↑↓, in which the electron spins are said to be paired. Here is a schematic orbital diagram for a hydrogen atom in its ground state:

From the orbital diagram, we can write the electron configuration in an abbreviated form in which the occupied orbitals are identified by their principal quantum number n and their value of l (s, p, d, or f), with the number of electrons in the subshell indicated by a superscript. For hydrogen, therefore, the single electron is placed in the 1s orbital, which is the orbital lowest in energy (Figure \(\PageIndex{1}\)), and the electron configuration is written as 1s1 and read as “one-s-one.”
A neutral helium atom, with an atomic number of 2 (Z = 2), has two electrons. We place one electron in the orbital that is lowest in energy, the 1s orbital. From the Pauli exclusion principle, we know that an orbital can contain two electrons with opposite spin, so we place the second electron in the same orbital as the first but pointing down, so that the electrons are paired. The orbital diagram for the helium atom is therefore
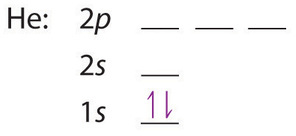
written as 1s2, where the superscript 2 implies the pairing of spins. Otherwise, our configuration would violate the Pauli principle.
The next element is lithium, with Z = 3 and three electrons in the neutral atom. We know that the 1s orbital can hold two of the electrons with their spins paired; the third electron must enter a higher energy orbital. Figure 6.29 tells us that the next lowest energy orbital is 2s, so the orbital diagram for lithium is

This electron configuration is written as 1s22s1.
The next element is beryllium, with Z = 4 and four electrons. We fill both the 1s and 2s orbitals to achieve a 1s22s2 electron configuration:
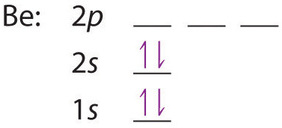
When we reach boron, with Z = 5 and five electrons, we must place the fifth electron in one of the 2p orbitals. Because all three 2p orbitals are degenerate, it doesn’t matter which one we select. The electron configuration of boron is 1s22s22p1:
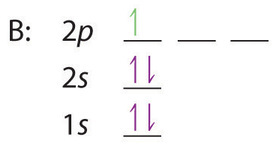
At carbon, with Z = 6 and six electrons, we are faced with a choice. Should the sixth electron be placed in the same 2p orbital that already has an electron, or should it go in one of the empty 2p orbitals? If it goes in an empty 2p orbital, will the sixth electron have its spin aligned with or be opposite to the spin of the fifth? In short, which of the following three orbital diagrams is correct for carbon, remembering that the 2p orbitals are degenerate?


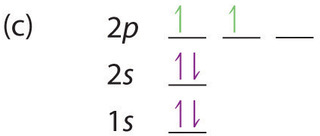
Because of electron-electron interactions, it is more favorable energetically for an electron to be in an unoccupied orbital than in one that is already occupied; hence we can eliminate choice a. Similarly, experiments have shown that choice b is slightly higher in energy (less stable) than choice c because electrons in degenerate orbitals prefer to line up with their spins parallel; thus, we can eliminate choice b. Choice c illustrates Hund’s rule (named after the German physicist Friedrich H. Hund, 1896–1997), which today says that the lowest-energy electron configuration for an atom is the one that has the maximum number of electrons with parallel spins in degenerate orbitals. By Hund’s rule, the electron configuration of carbon, which is 1s22s22p2, is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons.
When we get to nitrogen (Z = 7, with seven electrons), Hund’s rule tells us that the lowest-energy arrangement is

with three unpaired electrons. The electron configuration of nitrogen is thus 1s22s22p3.
At oxygen, with Z = 8 and eight electrons, we have no choice. One electron must be paired with another in one of the 2p orbitals, which gives us two unpaired electrons and a 1s22s22p4 electron configuration. Because all the 2p orbitals are degenerate, it doesn’t matter which one has the pair of electrons.
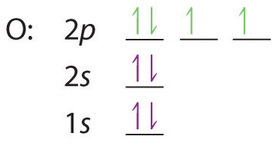
Similarly, fluorine has the electron configuration 1s22s22p5:
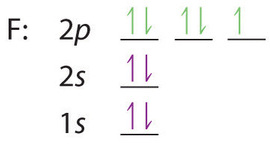
When we reach neon, with Z = 10, we have filled the 2p subshell, giving a 1s22s22p6 electron configuration:
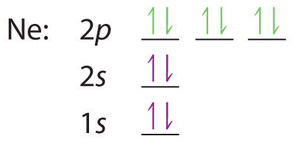
Notice that for neon, as for helium, all the orbitals through the 2p level are completely filled. This fact is very important in dictating both the chemical reactivity and the bonding of helium and neon, as you will see.
Example \(\PageIndex{1}\): Electronic Configuration of Phosphorus Atoms
Using Figure \(\PageIndex{2}\) as your guide, write the electron configuration of a neutral phosphorus atom. The atomic number of P is 15.
Solution
A neutral phosphorus atom has 15 electrons. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. That leaves 5 electrons. Of those 5 electrons, 2 can go into the 3s subshell, and the remaining 3 electrons can go into the 3p subshell. Thus, the electron configuration of neutral phosphorus atoms is 1s22s22p63s23p3.
Exercise \(\PageIndex{1}\): Electronic Configuration of Chlorine Atoms
Using Figure \(\PageIndex{2}\) as your guide, write the electron configuration of a neutral chlorine atom. The atomic number of Cl is 17.
- Answer
-
A neutral chlorine atom has 17 electrons. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. That leaves 7 electrons. Of those 7 electrons, 2 can go into the 3s subshell, and the remaining 5 electrons can go into the 3p subshell. Thus, the electron configuration of neutral chlorine atoms is 1s22s22p63s23p5.

