13.1.1: Effects of Temperature, Concentration, and Catalysts on Reaction Rates
- Page ID
- 443649
Learning Outcomes
- Describe how changing the temperature and concentration of a reaction affects the rate of a reaction.
- Define a catalyst and how a catalyst affects the rate of a reaction.
By their nature, some reactions occur very quickly, while others are very slow. However, certain changes in the reaction conditions can have an effect on the rate of a given chemical reaction. Collision theory can be utilized to explain these rate effects.
Effect of Temperature on Rate of Reaction
The rate of reaction was discussed in terms of three factors: collision frequency, the collision energy, and the geometric orientation. Remember that the collision frequency is the number of collisions per second. The collision frequency is dependent, among other factors, on the temperature of the reaction.
When the temperature is increased, the average velocity of the particles is increased. The average kinetic energy of these particles is also increased. The result is that the particles will collide more frequently, because the particles move around faster and will encounter more reactant particles. However, this is only a minor part of the reason why the rate is increased. Just because the particles are colliding more frequently does not mean that the reaction will definitely occur.
The major effect of increasing the temperature is that more of the particles that collide will have the amount of energy needed to have an effective collision. In other words, more particles will have the necessary activation energy.
At room temperature, the hydrogen and oxygen in the atmosphere do not have sufficient energy to attain the activation energy needed to produce water:
\[\ce{O_2} \left( g \right) + \ce{H_2} \left( g \right) \rightarrow \text{No reaction} \nonumber \]
At any one moment in the atmosphere, there are many collisions occurring between these two reactants. But what we find is that water is not formed from the oxygen and hydrogen molecules colliding in the atmosphere, because the activation energy barrier is just too high, and all the collisions are resulting in rebound. When we increase the temperature of the reactants or give them energy in some other way, the molecules have the necessary activation energy and are able to react to produce water:
\[\ce{O_2} \left( g \right) + \ce{H_2} \left( g \right) \rightarrow \ce{H_2O} \left( l \right) \nonumber \]
There are times when the rate of a reaction needs to be slowed down. Lowering the temperature could also be used to decrease the number of collisions that would occur and lowering the temperature would also reduce the kinetic energy available for activation energy. If the particles have insufficient activation energy, the collisions will result in rebound rather than reaction. Using this idea, when the rate of a reaction needs to be lower, keeping the particles from having sufficient activation energy will definitely keep the reaction at a lower rate.
Society uses the effects of temperature on reaction rate every day. Food storage is a prime example of how the temperature effect on reaction rate is utilized by society. Consumers store food in freezers and refrigerators to slow down the processes that cause it to spoil. The decrease in temperature decreases the rate at which food will break down or be broken down by bacteria. In the early years of the 20\(^\text{th}\) century, explorers were fascinated with being the first to reach the South Pole. In order to attempt such a difficult task at a time without most of the technology that we take for granted today, they devised a variety of ways of surviving. One method was to store their food in the snow to be used later during their advances to the pole. On some explorations, they buried so much food that they didn't need to use all of it, and some was left behind. Many years later, when this food was located and thawed, it was found to still be edible.
When milk, for example, is stored in the refrigerator, the molecules in the milk have less energy. This means that while molecules will still collide with other molecules, few of them will react (which means in this case "spoil") because the molecules do not have sufficient energy to overcome the activation energy barrier. The molecules do have energy and are colliding, however, and so, over time, even in the refrigerator, the milk will spoil. Eventually the higher energy molecules will gain the energy needed to react and when enough of these reactions occur, the milk becomes "soured".
However, if that same carton of milk was at room temperature, the milk would react (in other words, "spoil") much more quickly. Most of the molecules would have sufficient energy to overcome the energy barrier at room temperature, and many more collisions would occur. This allows for the milk to spoil in a fairly short amount of time. This is also the reason why most fruits and vegetables ripen in the summer when the temperature is much warmer. You may have experienced this first hand if you have ever bitten into an unripe banana—it was probably sour tasting and might even have felt like biting into a piece of wood! When a banana ripens, numerous reactions occur that produce all the compounds that we expect to taste in a banana. But this can only happen if the temperature is high enough to allow these reactions to make those products.
Effect of Concentration on Rate of Reaction
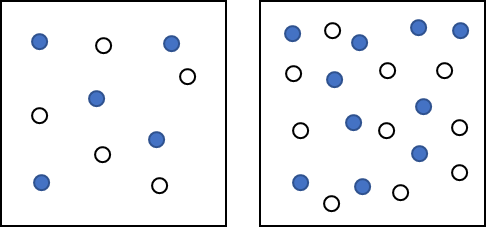
If you had an enclosed space, like a classroom, and there was one red ball and one green ball flying around the room in random motion, undergoing perfectly elastic collisions with the walls and with each other, in a given amount of time, the balls would collide with each other a certain number of times determined by probability. If you now put two red balls and one green ball in the room under the same conditions, the probability of a collision between a red ball and the green ball would exactly double. The green ball would have twice the chance of encountering a red ball in the same amount of time.
In terms of chemical reactions, a similar situation exists. Particles of two gaseous reactants or two reactants in solution have a certain probability of undergoing collisions with each other in a reaction vessel. If you double the concentration of either reactant, the probability of a collision doubles. The rate of reaction is proportional to the number of collisions per unit time. If one concentration is doubled, the number of collisions will also double. Assuming that the percent of collisions that are successful does not change, then having twice as many collisions will result in twice as many successful collisions. The rate of reaction is proportional to the number of collisions over time; increasing the concentration of either reactant increases the number of collisions, and therefore increases the number of successful collisions and the reaction rate.
For example, the chemical test used to identify a gas as oxygen, or not, relies on the fact that increasing the concentration of a reactant increases reaction rate. The reaction we call combustion refers to a reaction in which a flammable substance reacts with oxygen. If we light a wooden splint (a thin splinter of wood) on fire and then blow the fire out, the splint will continue to glow in air for a period of time. If we insert that glowing splint into any gas that does not contain oxygen, the splint will immediately cease to glow—that is, the reaction stops. Oxygen is the only gas that will support combustion, Air is approximately \(20\%\) oxygen gas. If we take that glowing splint and insert it into pure oxygen gas, the reaction will increase its rate by a factor of five, since pure oxygen has 5 times the concentration of oxygen that is in the air. When the reaction occurring on the glowing splint increases its rate by a factor of five, the glowing splint will suddenly burst back into full flame. This test, of thrusting a glowing splint into a gas, is used to identify the gas as oxygen. Only a greater concentration of oxygen than that found in air will cause the glowing splint to burst into flame.
Catalysts
The rates of some chemical reactions can be increased dramatically by introducing certain other substances into the reaction mixture. Hydrogen peroxide is used as a disinfectant for scrapes and cuts, and it can be found in many medicine cabinets as a \(3\%\) aqueous solution. Hydrogen peroxide naturally decomposes to produce water and oxygen gas, but the reaction is very slow. A bottle of hydrogen peroxide will last for several years before it needs to be replaced. However, the addition of just a small amount of manganese (IV) oxide to hydrogen peroxide will cause it to decompose completely in just a matter of minutes. A catalyst is a substance that increases the rate of a chemical reaction without being used up in the reaction. It accomplishes this task by providing an alternate reaction pathway that has a lower activation energy barrier. After the reaction occurs, a catalyst returns to its original state, so catalysts can be used over and over again. Because it is neither a reactant nor a product, a catalyst is shown in a chemical equation by being written above the yield arrow.
\[2 \ce{H_2O_2} \left( aq \right) \overset{\ce{MnO_2}}{\rightarrow} 2 \ce{H_2O} \left( l \right) + \ce{O_2} \left( g \right)\]
A catalyst works by changing the mechanism of the reaction, which can be thought of as the specific set of smaller steps by which the reactants become products. The important point is that the use of a catalyst lowers the overall activation energy of the reaction (see figure below). With a lower activation energy barrier, a greater percentage of reactant molecules are able to have effective collisions, and the reaction rate increases.

Catalysts are extremely important parts of many chemical reactions. Enzymes in your body act as nature's catalysts, allowing important biochemical reactions to occur at reasonable rates. Chemical companies constantly search for new and better catalysts to make reactions go faster and thus make the company more profitable.
Contributors and Attributions
Allison Soult, Ph.D. (Department of Chemistry, University of Kentucky)

