3.3: Quantum-Mechanical Orbitals and Electron Configurations
- Page ID
- 408971
- Represent the organization of electrons by an electron configuration and orbital diagram.
You now know that the periodic table is arranged in groups and periods (columns and rows) based on chemical and physical properties of the different elements. The first element, hydrogen (Z=1) has one proton and one electron and as you move right across the rows, each subsequent element has one additional proton and electron. You may have asked yourself, why are periodic trends observed across the rows and down the groups? Or, why do the rows have different numbers of elements, giving the table a unique shape?
These questions can be answered by learning more about the electrons in atoms. Although we have discussed the general arrangement of subatomic particles in atoms, we have said little about how electrons occupy the space around the nucleus. Do they move around the nucleus at random, or do they exist in some ordered arrangement?
In 1913, the Danish scientist Niels Bohr suggested that the electron in a hydrogen atom could not have any random energy, having only certain fixed values of energy that were indexed by the number n (now called a quantum number). Bohr suggested that the energy of the electron in hydrogen was quantized because it was in a specific orbit; much like the steps on a staircase does not have half or quarter stairs or the keys on a piano don't have notes in between, there are no energy levels in between each orbit. Figure
shows a model of the hydrogen atom based on Bohr's ideas.
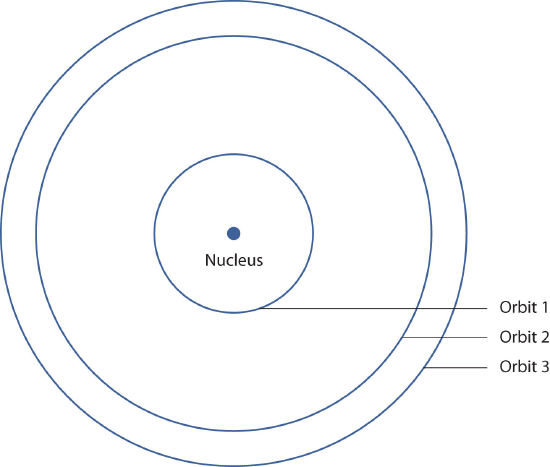
Figure \(\PageIndex{1}\): Bohr's Model of the Hydrogen Atom. Bohr's description of the hydrogen atom had specific orbits for the electron, which had quantized energies.
Bohr's ideas were useful, but were applicable only to the hydrogen atom. However, later researchers generalized Bohr's ideas into a new theory called quantum mechanics, which explains the behavior of electrons as if they were acting as a wave, not as particles. Quantum mechanics predicts two major things: quantized energies for electrons of all atoms (not just hydrogen) and an organization of electrons within atoms. Electrons are no longer thought of as being randomly distributed around a nucleus or restricted to certain orbits (in that regard, Bohr was wrong). Instead, electrons are collected into groups (shells) and subgroups (subshells) that explain much about the chemical behavior of the atom.
In the quantum-mechanical model of an atom, which is the modern and currently accepted model, the location of electrons in the atom are described by four quantum numbers, not just the one predicted by Bohr. Much like your home address can be used to locate you in a specific state, city, street, and house number, the first three quantum numbers identify approximately where electrons are in an atom. The fourth quantum number describes the electron and whether it is spin up or down (clockwise or counterclockwise). The theory and mathematics behind these four quantum numbers are well beyond the scope of this textbook, however, it is useful to learn some of the basics in order to understand how atoms behave and interact with (react) with other atoms.
Electron Arrangements: Shells, Subshells, and Orbitals
Electrons are organized according to their energies into sets called shells (labeled by the principle quantum number, n). Generally the higher the energy of a shell, the farther it is (on average) from the nucleus. Shells do not have specific, fixed distances from the nucleus, but an electron in a higher-energy shell will spend more time farther from the nucleus than does an electron in a lower-energy shell.
Shells are further divided into subsets of electrons called subshells, labeled by type as s, p, d, or f. The first shell has only one subshell, s. The second shell has two subshells, s and p; the third shell has three subshells, s, p, and d, and the fourth shell has four subshells, s, p, d, and f. Within each subshell, electrons are arranged into different numbers of orbitals, an s subshell is made up of one s orbital, a p subshell has two p orbitals, a d subshell, five d orbitals, and an f subshell, seven f orbitals. Each orbital has a different shape and orientation around the nucleus (Figure
, however, rather than representing an orbit, as the name suggests, orbitals define a boundary for the region of space where a given electron is most likely to be found. Lastly, a single orbital can hold up to two electrons each with a different spin.
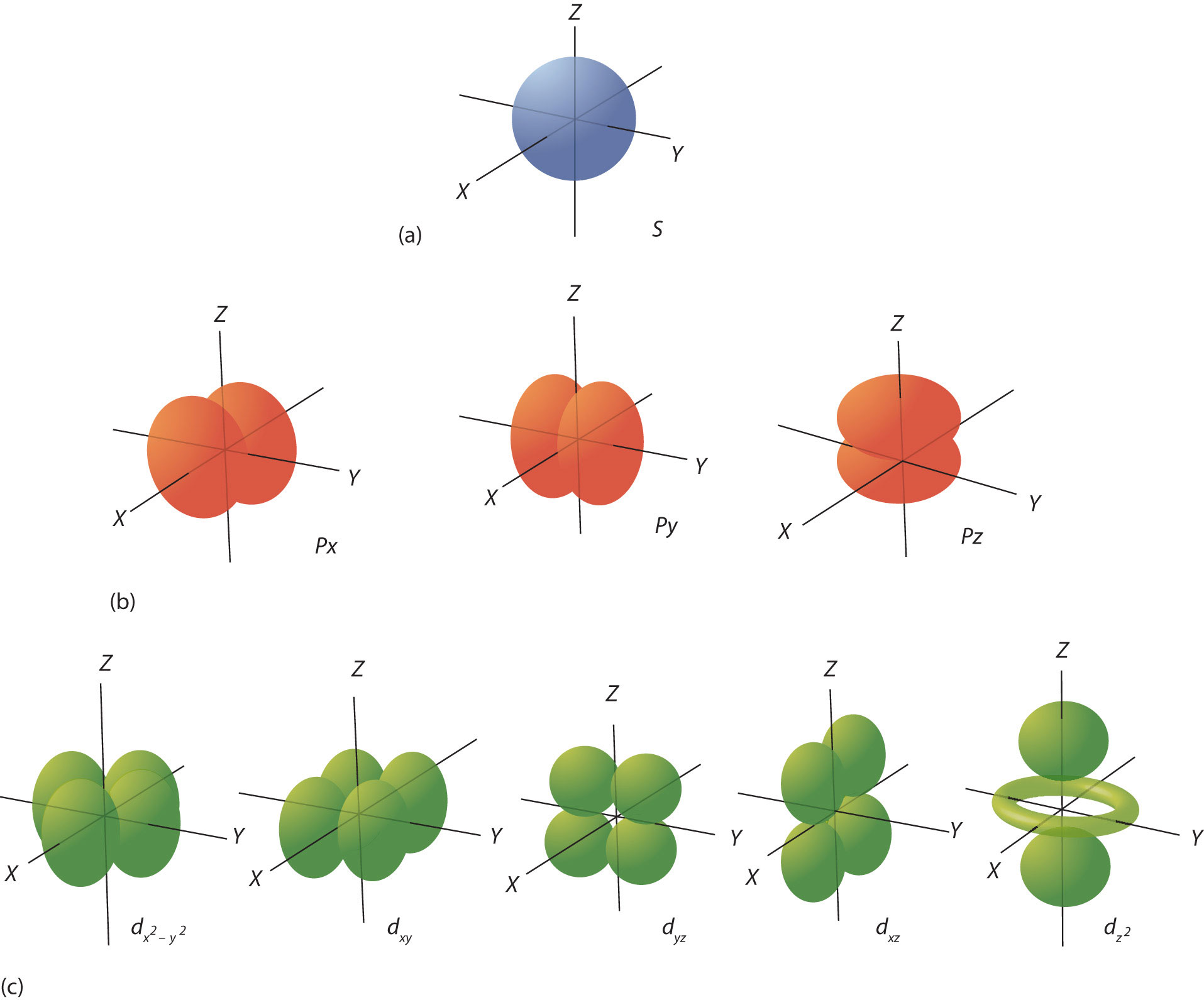
Figure \(\PageIndex{2}\): Electron Orbitals. (a) The lone s orbital in an s subshell is spherical in distribution. (b) The three p orbitals have two lobes, shaped kind of like dumbbells, each is oriented around the nucleus along a different axis. (c) The five d orbitals have four lobes, except for the orbital, which is a "dumbbell + torus" combination. They are all oriented in different directions.
It is important to note that according to quantum theory, there are specific allowed combinations of quantum numbers and others that are not allowed. For example, shell two can only have two subshells, s with one orbital and p with 3 orbitals, therefore, this shell can hold a maximum of eight electrons (four orbitals times two electrons each). It takes practice to learn the allowed combinations as shown in Table \(\PageIndex{1}\) but it is helpful to visualize the atom as a sphere with the nucleus in the center. Close to the nucleus, there is a smaller amount of space for electrons – a smaller shell. As the number of electrons increases, the shells that hold the electrons get larger and thus further away from the nucleus.
Table \(\PageIndex{1}\): Atomic shell and subshell structure with the number of electrons in each
| Shell | Number of Subshells | Names of Subshells | Number of Orbitals (per Subshell) | Number of Electrons (per Subshell) | Total Electrons (per Shell) |
|---|---|---|---|---|---|
| 1 | 1 | 1s | 1 | 2 | 2 |
| 2 | 2 | 2s and 2p | 1, 3 | 2, 6 | 8 |
| 3 | 3 | 3s, 3p, and 3d | 1, 3, 5 | 2, 6, 10 | 18 |
| 4 | 4 | 4s, 4p, 4d, and 4f | 1, 3, 5, 7 | 2, 6, 10, 14 | 32 |
All of this information about the shell, subshell, and orbital is put together to make up the "address" for an electron and all of the addresses for all the electrons in an atom make up the electron configuration, which is described more later.
Electron Configurations
Can you name one thing that easily distinguishes you from the rest of the world? And we're not talking about DNA—that's a little expensive to sequence. For many people, it is their email address. Your email address allows people all over the world to contact you. It does not belong to anyone else, but serves to identify you. Electrons also have a unique set of identifiers in the quantum numbers that describe their location and spin. Chemists use an electronic configuration to represent the organization of electrons in shells and subshells in an atom. An electron configuration simply lists the shell and subshell labels, with a right superscript giving the number of electrons in that subshell. The shells and subshells are listed in the order of filling. Electrons are typically organized around an atom by starting at the lowest possible quantum numbers first, which are the shells-subshells with lower energies.
For example, an H atom has a single electron in the 1s subshell. Its electron configuration is
H, 1s¹
He has two electrons in the 1s subshell. Its electron configuration is
He, 1s²
The three electrons for Li are arranged in the 1s subshell (two electrons) and the 2s subshell (one electron). The electron configuration of Li is
Li, 1s²2s¹
Be has four electrons, two in the 1s subshell and two in the 2s subshell. Its electron configuration is
Be, 1s²2s²
Now that the 2s subshell is filled, electrons in larger atoms must go into the 2p subshell, which can hold a maximum of six electrons. The next six elements progressively fill up the 2p subshell:
- B: 1s22s22p1
- C: 1s22s22p2
- N: 1s22s22p3
- O: 1s22s22p4
- F: 1s22s22p5
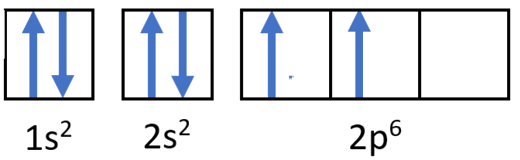
- Ne: 1s22s22p6
Now that the 2p subshell is filled (all possible subshells in the n = 2 shell), the next electron for the next-larger atom must go into the n = 3 shell, s subshell.
Second Period Elements
Periods refer to the horizontal rows of the periodic table. Looking at a periodic table you will see that the first period contains only the elements hydrogen and helium. This is because the first principal energy level consists of only the s sublevel and so only two electrons are required in order to fill the entire principal energy level. Each time a new principal energy level begins, as with the third element lithium, a new period is started on the periodic table. As one moves across the second period, electrons are successively added. With beryllium (Z=4), the 2s sublevel is complete and the 2p sublevel begins with boron (Z=5). Since there are three 2p orbitals and each orbital holds two electrons, the 2p sublevel is filled after six elements. Table \(\PageIndex{2}\) shows the electron configurations of the elements in the second period.
| Element Name | Symbol | Atomic Number | Electron Configuration |
|---|---|---|---|
| Lithium | Li | 3 | 1s² 2s¹ |
| Beryllium | Be | 4 | 1s² 2s² |
| Boron | B | 5 | 1s² 2s² 2p¹ |
| Carbon | C | 6 | 1s² 2s² 2p² |
| Nitrogen | N | 7 | 1s² 2s² 2p³ |
| Oxygen | O | 8 | 1s² 2s² 2p⁴ |
| Fluorine | F | 9 | 1s² 2s² 2p⁵ |
| Neon | Ne | 10 | 1s² 2s² 2p⁶ |
Aufbau Principle
Construction of a building begins at the bottom. The foundation is laid and the building goes up step by step. You obviously cannot start with the roof since there is no place to hang it. The building goes from the lowest level to the highest level in a systematic way. In order to create ground state electron configurations for any element, it is necessary to know the way in which the atomic sublevels are organized in order of increasing energy. Figure \(\PageIndex{4}\) shows the order of increasing energy of the sublevels.
The lowest energy sublevel is always the \(1s\) sublevel, which consists of one orbital. The single electron of the hydrogen atom will occupy the \(1s\) orbital when the atom is in its ground state. As we proceed with atoms with multiple electrons, those electrons are added to the next lowest sublevel: \(2s\), \(2p\), \(3s\), and so on. The Aufbau principle states that an electron occupies orbitals in order from lowest energy to highest. The Aufbau (German: "building up, construction") principle is sometimes referred to as the "building up" principle. It is worth noting that in reality atoms are not built by adding protons and electrons one at a time, and that this method is merely an aid for us to understand the end result.
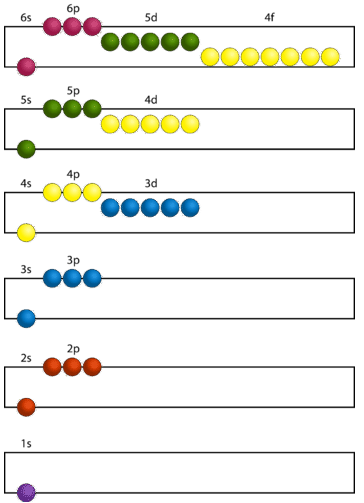
As seen in the figure above, the energies of the sublevels in different principal energy levels eventually begin to overlap. After the \(3p\) sublevel, it would seem logical that the \(3d\) sublevel should be the next lowest in energy. However, the \(4s\) sublevel is slightly lower in energy than the \(3d\) sublevel and thus fills first. Following the filling of the \(3d\) sublevel is the \(4p\), then the \(5s\) and the \(4d\). Note that the \(4f\) sublevel does not fill until just after the \(6s\) sublevel. Figure \(\PageIndex{6}\) is a useful and simple aid for keeping track of the order of fill of the atomic sublevels.
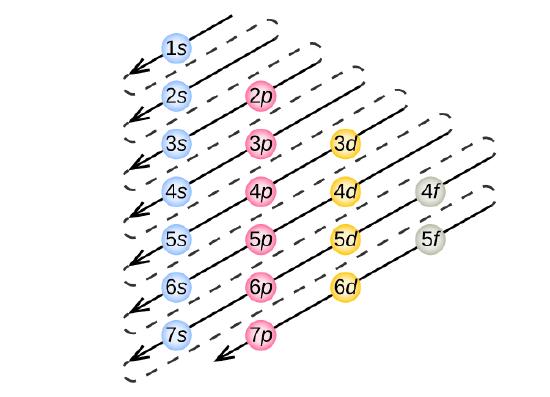
Nitrogen has 7 electrons. Write the electron configuration for nitrogen.
Solution:
Take a close look at Figure \(\PageIndex{5}\), and use it to figure out how many electrons go into each sublevel, and also the order in which the different sublevels get filled.
1. Begin by filling up the 1s sublevel. This gives 1s2. Now all of the orbitals in the red n = 1 block are filled.
- Since we used 2 electrons, there are 7 − 2 = 5 electrons left
2. Next, fill the 2s sublevel. This gives 1s22s2. Now all of the orbitals in the s sublevel of the orange n = 2 block are filled.
- Since we used another 2 electrons, there are 5 − 2 = 3 electrons left
3. Notice that we haven't filled the entire n = 2 block yet… there are still the p orbitals!
- The final 3 electrons go into the 2p sublevel. This gives 1s22s22p3
The overall electron configuration is: 1s22s22p3.
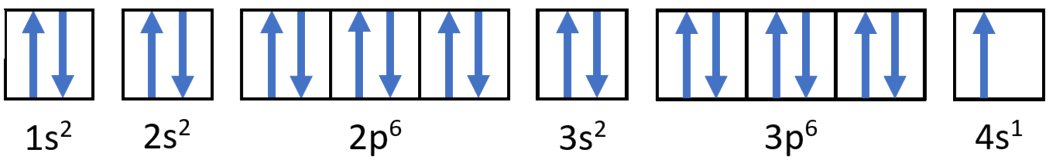
Potassium has 19 electrons. Write the electron configuration code for potassium.
Solution
This time, take a close look at Figure \(\PageIndex{5}\).
1. Begin by filling up the 1s sublevel. This gives 1s2. Now the n = 1 level is filled.
- Since we used 2 electrons, there are 19 − 2 = 17 electrons left
2. Next, fill the 2s sublevel. This gives 1s22s2
- Since we used another 2 electrons, there are 17 − 2 = 15 electrons left
3. Next, fill the 2p sublevel. This gives 1s22s22p6. Now the n = 2 level is filled.
- Since we used another 6 electrons, there are 15 − 6 = 9 electrons left
4. Next, fill the 3s sublevel. This gives 1s22s22p63s2
- Since we used another 2 electrons, there are 9 − 2 = 7 electrons left
5. Next, fill the 3p sublevel. This gives 1s22s22p63s23p6
- Since we used another 6 electrons, there are 7 − 6 = 1 electron left
Here's where we have to be careful – right after 3p6!
- Remember, 4s comes before 3d
6. The final electron goes into the 4s sublevel. This gives 1s22s22p63s23p64s1
The overall electron configuration is: 1s22s22p63s23p64s1
What is the electron configuration for Mg and Na?
- Answer Mg
- Mg: 1s22s22p63s2
- Answer Na
- Na: 1s22s22p63s1
Pauli Exclusion Principle
When we look at the orbital possibilities for a given atom, we see that there are different arrangements of electrons for each different type of atom. Since each electron must maintain its unique identity, we intuitively sense that the four quantum numbers for any given electron must not match up exactly with the four quantum numbers for any other electron in that atom.
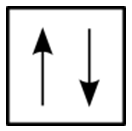
For the hydrogen atom, there is no problem since there is only one electron in the \(\ce{H}\) atom. However, when we get to helium we see that the first three quantum numbers for the two electrons are the same: same energy level, same spherical shape. What differentiates the two helium electrons is their spin. One of the electrons has a \(+\frac{1}{2}\) spin while the other electron has a \(-\frac{1}{2}\) spin. So the two electrons in the \(1s\) orbital are each unique and distinct from one another because their spins are different. This observation leads to the Pauli exclusion principle, which states that no two electrons in an atom can have the same set of four quantum numbers. The energy of the electron is specified by the principal, angular momentum, and magnetic quantum numbers. If those three numbers are identical for two electrons, the spin numbers must be different in order for the two electrons to be differentiated from one another. The two values of the spin quantum number allow each orbital to hold two electrons. Figure \(\PageIndex{7}\) shows how the electrons are indicated in a diagram.
Hund's Rule
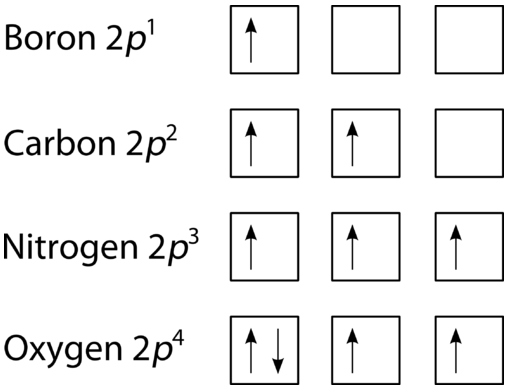
The last of the three rules for constructing electron arrangements requires electrons to be placed one at a time in a set of orbitals within the same sublevel. This minimizes the natural repulsive forces that one electron has for another. Hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each of the single electrons must have the same spin. The figure below shows how a set of three \(p\) orbitals is filled with one, two, three, and four electrons.
Orbital Filling Diagrams
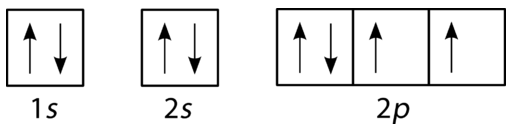
An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. Each sublevel is labeled by its principal energy level and sublevel. Electrons are indicated by arrows inside of the circles. An arrow pointing upwards indicates one spin direction, while a downward pointing arrow indicates the other direction. The orbital filling diagrams for hydrogen, helium, and lithium are shown in the figure below.
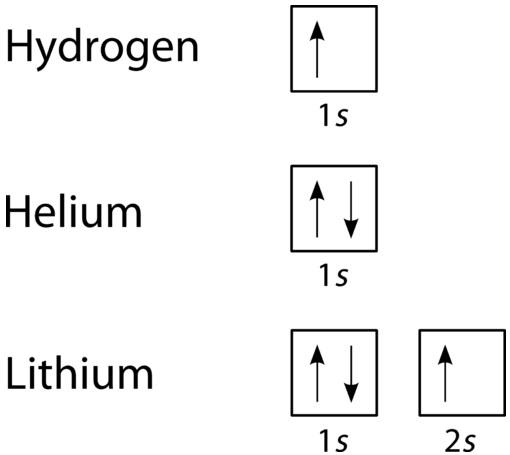
According to the Aufbau process, sublevels and orbitals are filled with electrons in order of increasing energy. Since the \(s\) sublevel consists of just one orbital, the second electron simply pairs up with the first electron as in helium. The next element is lithium and necessitates the use of the next available sublevel, the \(2s\).
The filling diagram for carbon is shown in Figure \(\PageIndex{10}\). There are two \(2p\) electrons for carbon and each occupies its own \(2p\) orbital.
Oxygen has four \(2p\) electrons. After each \(2p\) orbital has one electron in it, the fourth electron can be placed in the first \(2p\) orbital with a spin opposite that of the other electron in that orbital.
If you keep your papers in manila folders, you can pick up a folder and see how much it weighs. If you want to know how many different papers (articles, bank records, or whatever else you keep in a folder), you have to take everything out and count. A computer directory, on the other hand, tells you exactly how much you have in each file. We can get the same information on atoms. If we use an orbital filling diagram, we have to count arrows. When we look at electron configuration data, we simply add up the numbers.
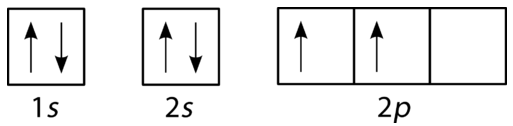
Draw the orbital filling diagram for carbon and write its electron configuration.
Solution
Step 1: List the known quantities and plan the problem.
Known
- Atomic number of carbon, Z=6
Use the order of fill diagram to draw an orbital filling diagram with a total of six electrons. Follow Hund's rule. Write the electron configuration.
Step 2: Construct the diagram.
Orbital filling diagram for carbon.
Electron configuration 1s22s22p2
Step 3: Think about your result.
Following the 2s sublevel is the 2p, and p sublevels always consist of three orbitals. All three orbitals need to be drawn even if one or more is unoccupied. According to Hund's rule, the sixth electron enters the second of those p orbitals and has the same spin as the fifth electron.
Write the electron configurations and orbital diagrams for
- Potassium atom: \(\ce{K}\)
- Arsenic atom: \(\ce{As}\)
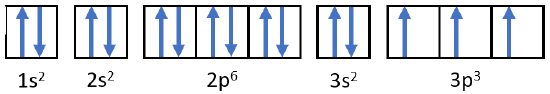
- Phosphorus atom: \(\ce{P}\)
- Answer a:
-
Potassium: \(1s^2 2s^2 2p^6 3s^2 3p^6 4s^1\)
- Answer b:
-
Arsenic: \(1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^{10} 4p^3\)

- Answer c:
-
Phosphorus \(1s^2 2s^2 2p^6 3s^2 3p^3\)
Summary
There are four different classes of electron orbitals. These orbitals are determined by the value of the angular momentum quantum number ℓ. An orbital is a wave function for an electron defined by the three quantum numbers, n, ℓ and mℓ. Orbitals define regions in space where you are likely to find electrons. s orbitals (ℓ = 0) are spherical shaped. p orbitals (ℓ = 1) are dumb-bell shaped. The three possible p orbitals are always perpendicular to each other.
Electron configuration notation simplifies the indication of where electrons are located in a specific atom. Superscripts are used to indicate the number of electrons in a given sublevel. The Aufbau principle gives the order of electron filling in an atom. It can be used to describe the locations and energy levels of every electron in a given atom. Hund's rule specifies the order of electron filling within a set of orbitals. Orbital filling diagrams are a way of indicating electron locations in orbitals. The Pauli exclusion principle specifies limits on how identical quantum numbers can be for two electrons in the same atom.
Vocabulary
- principal quantum number (n)
- Defines the energy level of the wave function for an electron, the size of the electron's standing wave, and the number of nodes in that wave.
- quantum numbers
- Integer numbers assigned to certain quantities in the electron wave function. Because electron standing waves must be continuous and must not "double over" on themselves, quantum numbers are restricted to integer values.
Contributions & Attributions
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
Henry Agnew (UC Davis)
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).


