8.5: Enthalpy of reactions
- Page ID
- 369493
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- To understand how enthalpy pertains to chemical reactions
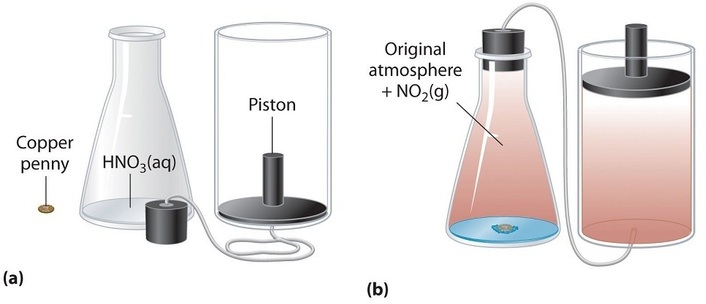
We have stated that the change in energy (\(ΔU\)) is equal to the sum of the heat produced and the work performed. Work done by an expanding gas is called pressure-volume work, (or just \(PV\) work). Consider, for example, a reaction that produces a gas, such as dissolving a piece of copper in concentrated nitric acid. The chemical equation for this reaction is as follows:
\[ \ce{Cu(s) + 4HNO3(aq) \rightarrow Cu(NO3)2(aq) + 2H_2O(l) + 2NO2(g)} \nonumber\]
If the reaction is carried out in a closed system that is maintained at constant pressure by a movable piston, the piston will rise as nitrogen dioxide gas is formed (Figure \(\PageIndex{1}\)). The system is performing work by lifting the piston against the downward force exerted by the atmosphere (i.e., atmospheric pressure). We find the amount of \(PV\) work done by multiplying the external pressure \(P\) by the change in volume caused by movement of the piston (\(ΔV\)). At a constant external pressure (here, atmospheric pressure),
\[w = −PΔV \label{5.4.2}\]
The negative sign associated with \(PV\) work done indicates that the system loses energy when the volume increases. If the volume increases at constant pressure (\(ΔV > 0\)), the work done by the system is negative, indicating that a system has lost energy by performing work on its surroundings. Conversely, if the volume decreases (\(ΔV < 0\)), the work done by the system is positive, which means that the surroundings have performed work on the system, thereby increasing its energy.
The internal energy \(U\) of a system is the sum of the kinetic energy and potential energy of all its components. It is the change in internal energy that produces heat plus work. To measure the energy changes that occur in chemical reactions, chemists usually use a related thermodynamic quantity called enthalpy (\(H\)) (from the Greek enthalpein, meaning “to warm”). The enthalpy of a system is defined as the sum of its internal energy \(U\) plus the product of its pressure \(P\) and volume \(V\):
\[H =U + PV \label{5.4.3}\]
Because internal energy, pressure, and volume are all state functions, enthalpy is also a state function. So we can define a change in enthalpy (\(\Delta H\)) accordingly
\[ΔH = H_{final} − H_{initial} \nonumber\]
If a chemical change occurs at constant pressure (i.e., for a given \(P\), \(ΔP = 0\)), the change in enthalpy (\(ΔH\)) is
\[ \begin{align} ΔH &= Δ(U + PV) \\[4pt] &= ΔU + ΔPV \\[4pt] &= ΔU + PΔV \label{5.4.4} \end{align} \]
Substituting \(q + w\) for \(ΔU\) (First Law of Thermodynamics) and \(−w\) for \(PΔV\) (Equation \(\ref{5.4.2}\)) into Equation \(\ref{5.4.4}\), we obtain
\[ \begin{align} ΔH &= ΔU + PΔV \\[4pt] &= q_p + \cancel{w} −\cancel{w} \\[4pt] &= q_p \label{5.4.5} \end{align} \]
The subscript \(p\) is used here to emphasize that this equation is true only for a process that occurs at constant pressure. From Equation \(\ref{5.4.5}\) we see that at constant pressure the change in enthalpy, \(ΔH\) of the system, is equal to the heat gained or lost.
\[ \begin{align} ΔH &= H_{final} − H_{initial} \\[4pt] &= q_p \label{5.4.6} \end{align} \]
Just as with \(ΔU\), because enthalpy is a state function, the magnitude of \(ΔH\) depends on only the initial and final states of the system, not on the path taken. Most important, the enthalpy change is the same even if the process does not occur at constant pressure.
To find \(ΔH\) for a reaction, measure \(q_p\).
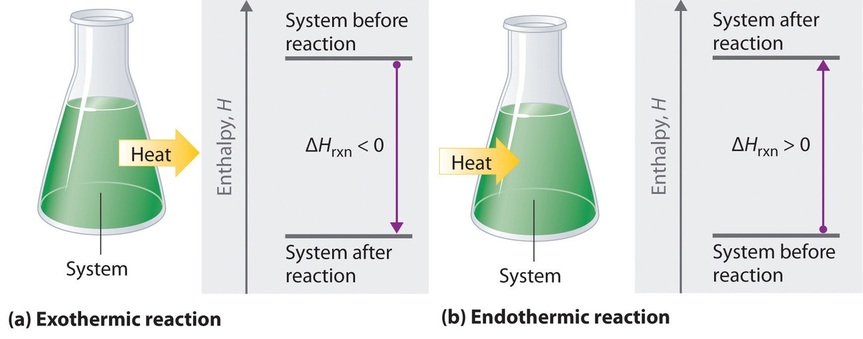
When we study energy changes in chemical reactions, the most important quantity is usually the enthalpy of reaction (\(ΔH_{rxn}\)), the change in enthalpy that occurs during a reaction (such as the dissolution of a piece of copper in nitric acid). If heat flows from a system to its surroundings, the enthalpy of the system decreases, so \(ΔH_{rxn}\) is negative. Conversely, if heat flows from the surroundings to a system, the enthalpy of the system increases, so \(ΔH_{rxn}\) is positive. Thus:
- \(ΔH_{rxn} < 0\) for an exothermic reaction, and
- \(ΔH_{rxn} > 0\) for an endothermic reaction.
In chemical reactions, bond breaking requires an input of energy and is therefore an endothermic process, whereas bond making releases energy, which is an exothermic process. The sign conventions for heat flow and enthalpy changes are summarized in the following table:
| Reaction Type | q | ΔHrxn |
|---|---|---|
| exothermic | < 0 | < 0 (heat flows from a system to its surroundings) |
| endothermic | > 0 | > 0 (heat flows from the surroundings to a system) |
If ΔHrxn is negative, then the enthalpy of the products is less than the enthalpy of the reactants; that is, an exothermic reaction is energetically downhill (Figure \(\PageIndex{2}a\)). Conversely, if ΔHrxn is positive, then the enthalpy of the products is greater than the enthalpy of the reactants; thus, an endothermic reaction is energetically uphill (Figure \(\PageIndex{2b}\)). Two important characteristics of enthalpy and changes in enthalpy are summarized in the following discussion.
Bond breaking ALWAYS requires an input of energy; bond making ALWAYS releases energy.y.
- Reversing a reaction or a process changes the sign of ΔH. Ice absorbs heat when it melts (electrostatic interactions are broken), so liquid water must release heat when it freezes (electrostatic interactions are formed):
\( \begin{matrix}
heat+ H_{2}O(s) \rightarrow H_{2}O(l) & \Delta H > 0
\end{matrix} \label{5.4.7} \)\( \begin{matrix}
H_{2}O(l) \rightarrow H_{2}O(s) + heat & \Delta H < 0
\end{matrix} \label{5.4.8} \)In both cases, the magnitude of the enthalpy change is the same; only the sign is different.
- Enthalpy is an extensive property (like mass). The magnitude of \(ΔH\) for a reaction is proportional to the amounts of the substances that react. For example, a large fire produces more heat than a single match, even though the chemical reaction—the combustion of wood—is the same in both cases. For this reason, the enthalpy change for a reaction is usually given in kilojoules per mole of a particular reactant or product. Consider Equation \(\ref{5.4.9}\), which describes the reaction of aluminum with iron(III) oxide (Fe2O3) at constant pressure. According to the reaction stoichiometry, 2 mol of Fe, 1 mol of Al2O3, and 851.5 kJ of heat are produced for every 2 mol of Al and 1 mol of Fe2O3 consumed:
\[ \ce{2Al(s) + Fe2O3(s) -> 2Fe (s) + Al2O3 (s) } + 815.5 \; kJ \label{5.4.9} \]
Thus ΔH = −851.5 kJ/mol of Fe2O3. We can also describe ΔH for the reaction as −425.8 kJ/mol of Al: because 2 mol of Al are consumed in the balanced chemical equation, we divide −851.5 kJ by 2. When a value for ΔH, in kilojoules rather than kilojoules per mole, is written after the reaction, as in Equation \(\ref{5.4.10}\), it is the value of ΔH corresponding to the reaction of the molar quantities of reactants as given in the balanced chemical equation:
\[ \ce{ 2Al(s) + Fe2O3(s) -> 2Fe(s) + Al2O3 (s)} \quad \Delta H_{rxn}= - 851.5 \; kJ \label{5.4.10} \]
If 4 mol of Al and 2 mol of \(\ce{Fe2O3}\) react, the change in enthalpy is 2 × (−851.5 kJ) = −1703 kJ. We can summarize the relationship between the amount of each substance and the enthalpy change for this reaction as follows:
\[ - \dfrac{851.5 \; kJ}{2 \; mol \;Al} = - \dfrac{425.8 \; kJ}{1 \; mol \;Al} = - \dfrac{1703 \; kJ}{4 \; mol \; Al} \label{5.4.6a} \]
The relationship between the magnitude of the enthalpy change and the mass of reactants is illustrated in Example \(\PageIndex{1}\).
Certain parts of the world, such as southern California and Saudi Arabia, are short of freshwater for drinking. One possible solution to the problem is to tow icebergs from Antarctica and then melt them as needed. If \(ΔH\) is 6.01 kJ/mol for the reaction at 0°C and constant pressure:
\[\ce{H2O(s) → H_2O(l)} \nonumber\]
How much energy would be required to melt a moderately large iceberg with a mass of 1.00 million metric tons (1.00 × 106 metric tons)? (A metric ton is 1000 kg.)
Given: energy per mole of ice and mass of iceberg
Asked for: energy required to melt iceberg
Strategy:
- Calculate the number of moles of ice contained in 1 million metric tons (1.00 × 106 metric tons) of ice.
- Calculate the energy needed to melt the ice by multiplying the number of moles of ice in the iceberg by the amount of energy required to melt 1 mol of ice.
Solution:
A Because enthalpy is an extensive property, the amount of energy required to melt ice depends on the amount of ice present. We are given ΔH for the process—that is, the amount of energy needed to melt 1 mol (or 18.015 g) of ice—so we need to calculate the number of moles of ice in the iceberg and multiply that number by ΔH (+6.01 kJ/mol):
\[ \begin{align*} moles \; H_{2}O & = 1.00\times 10^{6} \; \cancel{\text{metric ton }} \ce{H2O} \left ( \dfrac{1000 \; \cancel{kg}}{1 \; \cancel{\text{metric ton}}} \right ) \left ( \dfrac{1000 \; \cancel{g}}{1 \; \cancel{kg}} \right ) \left ( \dfrac{1 \; mol \; H_{2}O}{18.015 \; \cancel{g \; H_{2}O}} \right ) \\[4pt] & = 5.55\times 10^{10} \; mol \,\ce{H2O} \end{align*} \]
B The energy needed to melt the iceberg is thus
\[ \left ( \dfrac{6.01 \; kJ}{\cancel{mol \; H_{2}O}} \right )\left ( 5.55 \times 10^{10} \; \cancel{mol \; H_{2}O} \right )= 3.34 \times 10^{11} \; kJ \nonumber \]
Because so much energy is needed to melt the iceberg, this plan would require a relatively inexpensive source of energy to be practical. To give you some idea of the scale of such an operation, the amounts of different energy sources equivalent to the amount of energy needed to melt the iceberg are shown below.
Possible sources of the approximately \(3.34 \times 10^{11}\, kJ\) needed to melt a \(1.00 \times 10^6\) metric ton iceberg
- Combustion of 3.8 × 103 ft3 of natural gas
- Combustion of 68,000 barrels of oil
- Combustion of 15,000 tons of coal
- \(1.1 \times 10^8\) kilowatt-hours of electricity
Alternatively, we can rely on ambient temperatures to slowly melt the iceberg. The main issue with this idea is the cost of dragging the iceberg to the desired place.
If 17.3 g of powdered aluminum are allowed to react with excess \(\ce{Fe2O3}\), how much heat is produced?
- Answer
-
273 kJ
Enthalpies of Reaction
One way to report the heat absorbed or released would be to compile a massive set of reference tables that list the enthalpy changes for all possible chemical reactions, which would require an incredible amount of effort. Fortunately, since enthalpy is a state function, all we have to know is the initial and final states of the reaction. This allows us to calculate the enthalpy change for virtually any conceivable chemical reaction using a relatively small set of tabulated data, such as the following:
- Enthalpy of combustion (ΔHcomb) The change in enthalpy that occurs during a combustion reaction. Enthalpy changes have been measured for the combustion of virtually any substance that will burn in oxygen; these values are usually reported as the enthalpy of combustion per mole of substance.
- Enthalpy of fusion (ΔHfus) The enthalpy change that acompanies the melting (fusion) of 1 mol of a substance. The enthalpy change that accompanies the melting, or fusion, of 1 mol of a substance; these values have been measured for almost all the elements and for most simple compounds.
- Enthalpy of vaporization (ΔHvap) The enthalpy change that accompanies the vaporization of 1 mol of a substance. The enthalpy change that accompanies the vaporization of 1 mol of a substance; these values have also been measured for nearly all the elements and for most volatile compounds.
- Enthalpy of solution (ΔHsoln) The change in enthalpy that occurs when a specified amount of solute dissolves in a given quantity of solvent. The enthalpy change when a specified amount of solute dissolves in a given quantity of solvent.
| Substance | ΔHvap (kJ/mol) | ΔHfus (kJ/mol) |
|---|---|---|
| argon (Ar) | 6.3 | 1.3 |
| methane (CH4) | 9.2 | 0.84 |
| ethanol (CH3CH2OH) | 39.3 | 7.6 |
| benzene (C6H6) | 31.0 | 10.9 |
| water (H2O) | 40.7 | 6.0 |
| mercury (Hg) | 59.0 | 2.29 |
| iron (Fe) | 340 | 14 |
The sign convention is the same for all enthalpy changes: negative if heat is released by the system and positive if heat is absorbed by the system.
Enthalpy of Reaction: https://youtu.be/z2KUaIEF9qI
Enthalpy of Combustion
Standard enthalpy of combustion (\(ΔH_C^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines vigorously with oxygen) under standard state conditions; it is sometimes called “heat of combustion.” For example, the enthalpy of combustion of ethanol, −1366.8 kJ/mol, is the amount of heat produced when one mole of ethanol undergoes complete combustion at 25 °C and 1 atmosphere pressure, yielding products also at 25 °C and 1 atm.
\[\ce{C2H5OH}(l)+\ce{3O2}(g)⟶\ce{2CO2}+\ce{3H2O}(l)\hspace{20px}ΔH_{298}^\circ=\mathrm{−1366.8\: kJ} \label{5.4.8}\]
Enthalpies of combustion for many substances have been measured; a few of these are listed in Table \(\PageIndex{1}\). Many readily available substances with large enthalpies of combustion are used as fuels, including hydrogen, carbon (as coal or charcoal), and hydrocarbons (compounds containing only hydrogen and carbon), such as methane, propane, and the major components of gasoline.
| Substance | Combustion Reaction | Enthalpy of Combustion \(ΔH_c^\circ \left(\mathrm{\dfrac{kJ}{mol} \:at\:25°C}\right)\) |
|---|---|---|
| carbon | \(\ce{C}(s)+\ce{O2}(g)⟶\ce{CO2}(g)\) | −393.5 |
| hydrogen | \(\ce{H2}(g)+\frac{1}{2}\ce{O2}(g)⟶\ce{H2O}(l)\) | −285.8 |
| magnesium | \(\ce{Mg}(s)+\frac{1}{2}\ce{O2}(g)⟶\ce{MgO}(s)\) | −601.6 |
| sulfur | \(\ce{S}(s)+\ce{O2}(g)⟶\ce{SO2}(g)\) | −296.8 |
| carbon monoxide | \(\ce{CO}(g)+\frac{1}{2}\ce{O2}(g)⟶\ce{CO2}(g)\) | −283.0 |
| methane | \(\ce{CH4}(g)+\ce{2O2}(g)⟶\ce{CO2}(g)+\ce{2H2O}(l)\) | −890.8 |
| acetylene | \(\ce{C2H2}(g)+\dfrac{5}{2}\ce{O2}(g)⟶\ce{2CO2}(g)+\ce{H2O}(l)\) | −1301.1 |
| ethanol | \(\ce{C2H5OH}(l)+\ce{3O2}(g)⟶\ce{CO2}(g)+\ce{3H2O}(l)\) | −1366.8 |
| methanol | \(\ce{CH3OH}(l)+\dfrac{3}{2}\ce{O2}(g)⟶\ce{CO2}(g)+\ce{2H2O}(l)\) | −726.1 |
| isooctane | \(\ce{C8H18}(l)+\dfrac{25}{2}\ce{O2}(g)⟶\ce{8CO2}(g)+\ce{9H2O}(l)\) | −5461 |
As Figure \(\PageIndex{3}\) suggests, the combustion of gasoline is a highly exothermic process. Let us determine the approximate amount of heat produced by burning 1.00 L of gasoline, assuming the enthalpy of combustion of gasoline is the same as that of isooctane, a common component of gasoline. The density of isooctane is 0.692 g/mL.

Solution
Starting with a known amount (1.00 L of isooctane), we can perform conversions between units until we arrive at the desired amount of heat or energy. The enthalpy of combustion of isooctane provides one of the necessary conversions. Table \(\PageIndex{1}\) gives this value as −5460 kJ per 1 mole of isooctane (C8H18).
Using these data,
The combustion of 1.00 L of isooctane produces 33,100 kJ of heat. (This amount of energy is enough to melt 99.2 kg, or about 218 lbs, of ice.)
Note: If you do this calculation one step at a time, you would find:
\(\begin {align*}
&\mathrm{1.00\:L\:\ce{C8H18}⟶1.00×10^3\:mL\:\ce{C8H18}}\\
&\mathrm{1.00×10^3\:mL\:\ce{C8H18}⟶692\:g\:\ce{C8H18}}\\
&\mathrm{692\:g\:\ce{C8H18}⟶6.07\:mol\:\ce{C8H18}}\\
&\mathrm{692\:g\:\ce{C8H18}⟶−3.31×10^4\:kJ}
\end {align*}\)
How much heat is produced by the combustion of 125 g of acetylene?
- Answer
-
6.25 × 103 kJ
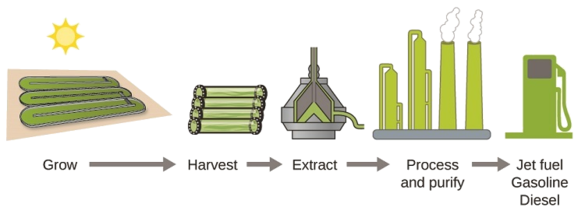
As reserves of fossil fuels diminish and become more costly to extract, the search is ongoing for replacement fuel sources for the future. Among the most promising biofuels are those derived from algae (Figure \(\PageIndex{4}\)). The species of algae used are nontoxic, biodegradable, and among the world’s fastest growing organisms. About 50% of algal weight is oil, which can be readily converted into fuel such as biodiesel. Algae can yield 26,000 gallons of biofuel per hectare—much more energy per acre than other crops. Some strains of algae can flourish in brackish water that is not usable for growing other crops. Algae can produce biodiesel, biogasoline, ethanol, butanol, methane, and even jet fuel.

According to the US Department of Energy, only 39,000 square kilometers (about 0.4% of the land mass of the US or less than \(\dfrac{1}{7}\) of the area used to grow corn) can produce enough algal fuel to replace all the petroleum-based fuel used in the US. The cost of algal fuels is becoming more competitive—for instance, the US Air Force is producing jet fuel from algae at a total cost of under $5 per gallon. The process used to produce algal fuel is as follows: grow the algae (which use sunlight as their energy source and CO2 as a raw material); harvest the algae; extract the fuel compounds (or precursor compounds); process as necessary (e.g., perform a transesterification reaction to make biodiesel); purify; and distribute (Figure \(\PageIndex{5}\)).
Summary
For a chemical reaction, the enthalpy of reaction (\(ΔH_{rxn}\)) is the difference in enthalpy between products and reactants; the units of \(ΔH_{rxn}\) are kilojoules per mole. Reversing a chemical reaction reverses the sign of \(ΔH_{rxn}\).
Contributors and Attributions
Modified by Joshua Halpern (Howard University)

