8.6: How Much of the Excess Reactant Remains after a Reaction
- Page ID
- 371650
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Identify the excess reactant in a given chemical reaction.
- Calculate how much excess reactant(s) remains when the reaction is complete.
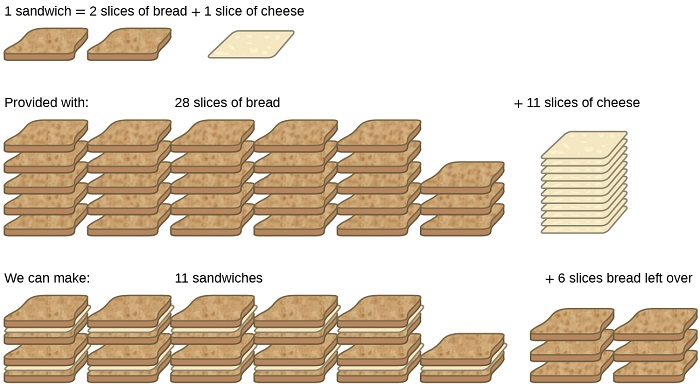
Consider the food analogy, making grilled cheese sandwiches (Figure \(\PageIndex{1}\)):
\[\text{1 slice of cheese} + \text{2 slices of bread} \rightarrow \text{1 sandwich} \label{4.5.A}\]
Stoichiometric amounts of sandwich ingredients for this recipe are bread and cheese slices in a 2:1 ratio. Provided with 28 slices of bread and 11 slices of cheese, one may prepare 11 sandwiches per the provided recipe, using all the provided cheese and having six slices of bread left over. In this scenario, the number of sandwiches prepared has been limited by the number of cheese slices, and six bread slices have been provided in excess.
Consider this concept now with regard to a chemical process, the reaction of hydrogen with chlorine to yield hydrogen chloride:
\[\ce{H2}(g) + \ce{Cl2}(g)\rightarrow \ce{2HCl}(g)\]
The balanced equation shows the hydrogen and chlorine react in a 1:1 stoichiometric ratio. If these reactants are provided in any other amounts, one of the reactants will nearly always be entirely consumed, thus limiting the amount of product that may be generated. This substance is the limiting reactant, and the other substance is the excess reactant. Identifying the limiting and excess reactants for a given situation requires computing the molar amounts of each reactant provided and comparing them to the stoichiometric amounts represented in the balanced chemical equation.
For example, imagine combining 6 moles of H2 and 4 moles of Cl2. Identifying the limiting reactant involves comparing the amount of product expected for the complete reaction of each reactant. Each reactant amount is used to separately calculate the amount of product that would be formed per the reaction’s stoichiometry. The reactant yielding the lesser amount of product is the limiting reactant.
For the example in the previous paragraph, the complete reaction of the hydrogen would yield
\[\mathrm{mol\: HCl\: produced=6\: mol\:H_2\times \dfrac{2\: mol\: HCl}{1\: mol\:H_2}=12\: mol\: HCl}\]
The complete reaction of the provided chlorine would produce
\[\mathrm{mol\: HCl\: produced=4\: mol\:Cl_2\times \dfrac{2\: mol\: HCl}{1\: mol\:Cl_2}=8\: mol\: HCl}\]
The chlorine will be completely consumed once 8 moles of HCl have been produced. Since enough hydrogen was provided to yield 12 moles of HCl, there will be non-reacted hydrogen remaining once this reaction is complete. Chlorine, therefore, is the limiting reactant and hydrogen is the excess reactant (Figure \(\PageIndex{2}\)). To determine the amount of excess reactant that remains, the amount of hydrogen consumed in the reaction can be subtracted from the initial quantity of hydrogen.
The amount of hydrogen consumed is
\[\mathrm{mol\: H_2\: produced=8\: mol\:HCl\times \dfrac{1\: mol\: H_2}{2\: mol\:HCl}=4\: mol\: H_2}\]
Subtract the hydrogen consumed from the starting quantity
\[\mathrm{mole\: of\: excess\:H_{2}=6\:mol\:H_{2}\:initial\:-\:4\:mol\:H_{2}\:consumed\:=\:2\:mol\:H_{2}\; excess}\]
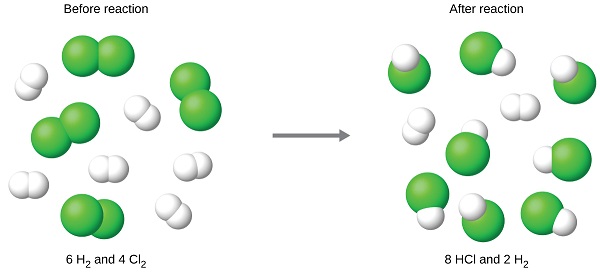
Figure \(\PageIndex{2}\): When H2 and Cl2 are combined in nonstoichiometric amounts, one of these reactants will limit the amount of HCl that can be produced. This illustration shows a reaction in which hydrogen is present in excess and chlorine is the limiting reactant.
Example \(\PageIndex{3}\): Limiting Reactant and Mass of Excess Reactant
5.00 g Rb are combined with 3.44 g MgCl2 according to the chemical reaction:
2 Rb (s) + MgCl2 (s) → Mg (s) + 2 RbCl (s)
- What mass of Mg is formed?
- What mass of which reactant is left over?
Solution
| Steps for Problem Solving | |
|---|---|
| Identify the "given" information and what the problem is asking you to "find." | Given: 5.00 g Rb reacted; 3.44 g MgCl2 reacted Find: theoretical yield Mg; identify excess reactant and its mass |
| List other known quantities. | 1 mol Rb = 85.47 g Rb 1 mol MgCl2 = 95.21 g MgCl2 1 mol Mg = 24.31 g Mg 2 mol Rb: 1 mol Mg 1 mol MgCl2: 1 mol Mg |
| Prepare concept maps using the proper conversion factor(s). | \({\color[rgb]{0.5, 0.0, 0.5}\boxed{\;\;\;\mathrm g\;\mathrm{Rb}\;\;\;}}\xrightarrow[{85.47\;\mathrm g\;\mathrm{Rb}}]{1\;\mathrm{mol}\;\mathrm{Rb}}{\color[rgb]{0.8, 0.0, 0.0}\boxed{\;\;\mathrm{mol}\;\mathrm{Rb}\;\;}}\xrightarrow[{2\;\mathrm{mol}\;\mathrm{Rb}}]{1\;\mathrm{mol}\;\mathrm{Mg}}{\color[rgb]{0.0, 0.0, 1.0}\boxed{\;\;\mathrm{mol}\;\mathrm{Mg}\;\;}}\xrightarrow[{1\;\mathrm{mol}\;\mathrm{Mg}}]{24.31\;\mathrm g\;\mathrm{Mg}}{\color[rgb]{0.0, 0.5, 0.0}\boxed{\;\;\;\mathrm g\;\mathrm{Mg}\;\;\;}}\) \({\color[rgb]{0.5, 0.0, 0.5}\boxed{\;\mathrm g\;{\mathrm{MgCl}}_2\;}}\xrightarrow[{95.21\;\mathrm g\;{\mathrm{MgCl}}_2}]{1\;\mathrm{mol}\;{\mathrm{MgCl}}_2}{\color[rgb]{0.8, 0.0, 0.0}\boxed{\mathrm{mol}\;{\mathrm{MgCl}}_2}}\xrightarrow[{2\;\mathrm{mol}\;{\mathrm{MgCl}}_2}]{1\;\mathrm{mol}\;\mathrm{Mg}}{\color[rgb]{0.0, 0.0, 1.0}\boxed{\;\;\mathrm{mol}\;\mathrm{Mg}\;\;}}\xrightarrow[{1\;\mathrm{mol}\;\mathrm{Mg}}]{24.31\;\mathrm g\;\mathrm{Mg}}{\color[rgb]{0.0, 0.5, 0.0}\boxed{\;\;\;\mathrm g\;\mathrm{Mg}\;\;\;}}\) |
| Calculate the theoretical yield. Select the smallest answer. |
\(5.00\:\cancel{\mathrm g\;\mathrm{Rb}}\times\dfrac{1\;\cancel{\mathrm{mol}\;\mathrm{Rb}}}{85.47\:\cancel{\mathrm g\;\mathrm{Rb}}}\times\dfrac{1\;\cancel{\mathrm{mol}\;\mathrm{Mg}}}{2\:\cancel{\mathrm{mol}\;\mathrm{Rb}}}\times\dfrac{24.31\;\mathrm g\;\mathrm{Mg}}{1\;\cancel{\mathrm{mol}\;\mathrm{Mg}}}=\boxed{0.711\;\mathrm g\;\mathrm{Mg}}\) \(3.44\:\cancel{\mathrm g\;{\mathrm{MgCl}}_2}\times\dfrac{1\;\cancel{\mathrm{mol}\;{\mathrm{MgCl}}_2}}{95.21\:\cancel{\mathrm g\;{\mathrm{MgCl}}_2}}\times\dfrac{1\;\cancel{\mathrm{mol}\;\mathrm{Mg}}}{1\:\cancel{\mathrm{mol}\;{\mathrm{MgCl}}_2}}\times\dfrac{24.31\;\mathrm g\;\mathrm{Mg}}{1\;\cancel{\mathrm{mol}\;\mathrm{Mg}}}=\xcancel{0.878\;\mathrm g\;\mathrm{Mg}}\) |
| Identify the limiting reactant(s) and excess reactant(s). | The limiting reactant is Rb since it would yield the least amount of product (0.711 g Mg). The excess reactant is MgCl2 since its complete reaction would have yielded up to 0.878 g Mg. |
| Calculate the mass of excess reactant that reacts. | Start with 0.711 g Mg, since it is the theoretical yield and the amount produced. \(0.711\:\cancel{\mathrm g\;\mathrm{Mg}}\times\dfrac{1\;\cancel{\mathrm{mol}\;\mathrm{Mg}}}{24.31\:\cancel{\mathrm g\;\mathrm{Mg}}}\times\dfrac{1\;\cancel{\mathrm{mol}\;{\mathrm{MgCl}}_2}}{1\;\cancel{\mathrm{mol}\;\mathrm{Mg}}}\times\dfrac{95.21\;\mathrm g\;{\mathrm{MgCl}}_2}{1\;\cancel{\mathrm{mol}\;{\mathrm{MgCl}}_2}}=\boxed{2.78\;\mathrm g\;{\mathrm{MgCl}}_2\;\mathrm{reacted}}\) |
| Calculate the mass of excess reactant that remains and think about the result. | We started with 3.44 g MgCl2 and found that 2.78 g MgCl2 reacted. This makes sense, since MgCl2 is the excess reactant. Less should react than we started with. 3.44 g MgCl2 initially – 2.78 g MgCl2 reacted = 0.66 g MgCl2 remain |
Exercise \(\PageIndex{1}\)
Given the balanced chemical equation:
2 C8H18 + 25 O2 → 16 CO2 + 18 H2O
- If 24.5 moles of C8H18 are reacted with 245 moles of O2, how many moles of CO2 are produced assuming complete reaction?
- How many moles of which reactant remain unconsumed?
- Answer A
- 157 mol CO2 are produced.
- Answer B
- 4.9 mol C8H18 remain unconsumed.
Exercise \(\PageIndex{2}\)
Potassium superoxide, KO2, is used in rebreathing gas masks to generate oxygen according to the equation below.
4 KO2 (s) + 2 H2O (l) → 4 KOH (s) + 3 O2 (g)
- What is the theoretical yield of oxygen, in units of grams, when 108 g of KO2 reacts with 16.8 g of H2O?
- Which reactant is the limiting reactant?
- Which reactant is present in excess?
- Answer A
- 36.5 g O2 are produced.
- Answer B
- The limiting reactant is KO2.
- Answer C
- H2O is present in excess.
Video Topics
A variation of limiting reactant problems involves determining how much of the non-limiting reactant remains after a reaction. This is done by generating mole ratio conversion unit created by from reaction's stoichiometry. By determining how much of the non-limiting reactant is used during the reaction the amount of excess can be determined.
Link to Video
How Much of the Excess Reactant Remains after a Reaction: https://youtu.be/K2II-QuBsKs
This project was preformed to supply Libretext authors with videos on General Chemistry topics which can be used to enhance their projects. Also, these videos are meant to act as a learning resource for all General Chemistry students.
Attribution
Prof. Steven Farmer (Sonoma State University)
- Sarick Shah (UCD)
-
Henry Agnew (UC Davis)
- Lance S. Lund (Anoka-Ramsey Community College)

