8.2: Mole-to-Mole Conversions
- Page ID
- 371646
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Use a balanced chemical equation to determine molar relationships between substances.
Previously, you learned to balance chemical equations by comparing the numbers of each type of atom in the reactants and products. The coefficients in front of the chemical formulas represent the numbers of molecules or formula units (depending on the type of substance). As follows, we will extend the meaning of the coefficients in a chemical equation.
Consider the simple chemical equation:
\[2H_2 + O_2 → 2H_2O\]
The convention for writing balanced chemical equations is to use the lowest whole-number ratio for the coefficients. However, the equation is balanced as long as the coefficients are in a 2:1:2 ratio. For example, this equation is also balanced if we write it as
\[4H_2 + 2O_2 → 4H_2O\]
The ratio of the coefficients is 4:2:4, which reduces to 2:1:2. The equation is also balanced if we were to write it as
\[22H_2 + 11O_2 → 22H_2O\]
because 22:11:22 also reduces to 2:1:2.
Suppose we want to use larger numbers. Consider the following coefficients:
\[12.044 \times 10^{23}\; \ce{H_2} + 6.022 \times 10^{23}\; \ce{O_2} → 12.044 \times 10^{23}\; \ce{H_2O} \nonumber\]
These coefficients also have the ratio 2:1:2 (check it and see), so this equation is balanced. But 6.022 × 1023 is 1 mol, while 12.044 × 1023 is 2 mol (and the number is written that way to make this more obvious), so we can simplify this version of the equation by writing it as
\[2 \;mol\; \ce{H_2} + 1\; mol\; \ce{O_2} → 2 \;mol\; \ce{H_2O} \nonumber\]
We can leave out the word mol and not write the 1 coefficient (as is our habit), so the final form of the equation, still balanced, is
\[\ce{2H_2 + O_2 → 2H_2O} \nonumber\]
Now we interpret the coefficients as referring to molar amounts, not individual molecules. The lesson? Balanced chemical equations are balanced not only at the molecular level, but also in terms of molar amounts of reactants and products. Thus, we can read this reaction as “two moles of hydrogen react with one mole of oxygen to produce two moles of water.”
By the same token, the ratios we constructed to describe a molecular reaction can also be constructed in terms of moles rather than molecules. For the reaction in which hydrogen and oxygen combine to make water, for example, we can construct the following ratios:
\[\mathrm{\dfrac{2\: mol\: H_2}{1\: mol\: O_2}\: or\: \dfrac{1\: mol\: O_2}{2\: mol\: H_2}}\]
\[\mathrm{\dfrac{2\: mol\: H_2O}{1\: mol\: O_2}\: or\: \dfrac{1\: mol\: O_2}{2\: mol\: H_2O}}\]
\[\mathrm{\dfrac{2\: mol\: H_2}{2\: mol\: H_2O}\: or\: \dfrac{2\: mol\: H_2O}{2\: mol\: H_2}}\]
We can use these ratios to determine what amount of a substance, in moles, will react with or produce a given number of moles of a different substance. The study of the numerical relationships between the reactants and the products in balanced chemical reactions is called stoichiometry.
Example \(\PageIndex{1}\)
How many moles of oxygen react with hydrogen to produce 27.6 mol of \(\ce{H2O}\)?
Solution
| Steps for Problem Solving | How many moles of oxygen react with hydrogen to produce 27.6 mol of \(\ce{H2O}\)? |
|---|---|
| Find a balanced equation that describes the reaction. |
Unbalanced: H2 + O2 → H2O Balanced: 2H2 + O2 → 2H2O |
| Identify the "given" information and what the problem is asking you to "find." | Given: moles H2O Find: moles oxygen |
| List other known quantities. | 1 mol O2 = 2 mol H2O |
|
Prepare a concept map and use the proper conversion factor. |
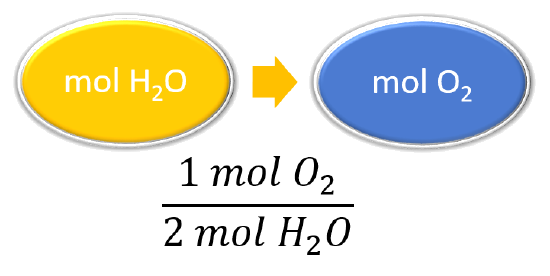 |
| Cancel units and calculate. |
\(\mathrm{\cancel{27.6\: mol\: H_2O}\times\dfrac{1\: mol\: O_2}{\cancel{2\: mol\: H_2O}}=13.8\: mol\: O_2}\) To produce 27.6 mol of H2O, 13.8 mol of O2 react. |
| Think about your result. | Since each mole of oxygen produces twice as many moles of water, it makes sense that the produced amount is greater than the reactant amount |
Example \(\PageIndex{2}\)
How many moles of ammonia are produced if 4.20 moles of hydrogen are reacted with an excess of nitrogen?
Solution
| Steps for Problem Solving | How many moles of ammonia are produced if 4.20 moles of hydrogen are reacted with an excess of nitrogen? |
|---|---|
| Find a balanced equation that describes the reaction. |
Unbalanced: N2 + H2 → NH3 Balanced: N2 + 3H2 → 2NH3 |
| Identify the "given" information and what the problem is asking you to "find." |
Given: \(\ce{H_2} = 4.20 \: \text{mol}\) Find: \(\text{mol}\) of \(\ce{NH_3}\) |
| List other known quantities. | 3 mol H2 = 2 mol NH3 |
|
Prepare a concept map and use the proper conversion factor. |
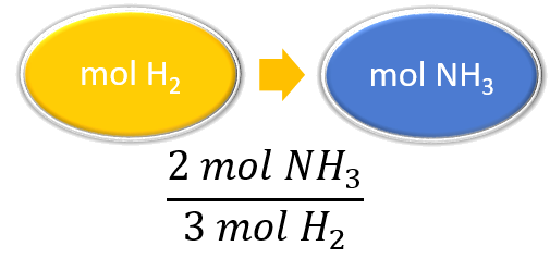 |
| Cancel units and calculate. |
\(\cancel{4.20 \: \text{mol} \: H_2} \times \dfrac{2 \: \text{mol} \: NH_3}{\cancel{3 \: \text{mol} \: H_2}} = 2.80 \: \text{mol} \: NH_3\) The reaction of \(4.20 \: \text{mol}\) of hydrogen with excess nitrogen produces \(2.80 \: \text{mol}\) of ammonia. |
| Think about your result. | The result corresponds to the 3:2 ratio of hydrogen to ammonia from the balanced equation. |
Exercise \(\PageIndex{3}\)
- Given the following balanced chemical equation: \[\ce{C5H12 + 8O2 → 5CO2 + 6H2O} \nonumber\], How many moles of \(\ce{H2O}\) can be formed if 0.0652 mol of \(\ce{C_{5}H_{12}}\) were to react?
- Balance the following unbalanced equation and determine how many moles of \(\ce{H2O}\) are produced when 1.65 mol of NH3 react: \[\ce{NH3 + O2 → N2 + H2O} \nonumber\]
- Answer a
- 0.391 mol H2O
- Answer b
- 4NH3 + 3O2 → 2N2 + 6H2O; 2.48 mol H2O
Summary
- The balanced chemical reaction can be used to determine molar relationships between substances.
Contributions & Attributions
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
Henry Agnew (UC Davis)

