5.6.8: Nuclear Bombs
- Page ID
- 466612
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe the key features of an atomic bomb.
- Define nuclear fallout.
World War II brought about many advances in weaponry. The Nazis used the immense scientific ability of their newly conquered countries to attempt to create super weapons to keep the new territory. The weapons included new tanks like the feared Tiger Tank, or the highly advanced Luftwaffe Air Force. However, the one weapon the Nazi's really wanted to hold was the atomic bomb. The problem was that many of the great minds they once had left the country and came to the United States. In the end, Allied troops got to Hitler and his scientists before they could complete any atomic bombs. The U.S. also wanted to create an atomic bomb, and started The Manhattan to accomplish this goal.
Unlike the Nazi's efforts, The Manhattan Project in the United States was successful in part due to the vast resources that could be pulled together. The great minds worked under the University of Chicago football field to try and create fission (a reaction where a neutron collides with the nucleus of an atom causing it to split into smaller particles). A plant in Tennessee had the sole purpose of producing enough uranium for atomic bomb testing. Another testing center was established in New Mexico, becoming the epicenter of the operation. This is where the bombs were constructed and tested. The resources needed were immense. There had to be enough fuel for the bomb, and the materials required to make it. There had to be housing and food for all the scientists. Maybe most importantly, there had to be enough money to support the project. After years of hard work, an atomic bomb test took place on July 16, 1945. The bomb was held together in some parts by just tape, yet it was a huge success.
At the time of the atomic bomb test, the war in Europe had already ended, however, the war in the Pacific continued. This conflict was a lot different than the war in Europe. In Europe, Allied forces landed once and fought their way through to Berlin to defeat the Nazi's. However, in the Pacific, Japan controlled hundreds of Islands between the United States and Japan, the number of invasions required would have cost large amounts of American lives and resources. President Truman, first proposed the Potsdam Declaration, warning of "prompt and utter destruction" if Japan did not agree. After 11 days, when there was no reply from Japan, President Truman authorized the use of the atomic bomb on Japan.
The first nuclear weapon used in combat, nicknamed "Little Boy," was dropped on Hiroshima on August 6, 1945, by the crew of the plane called the Enola Gay. The bomb destroyed the city, killing almost 100,000 people. The city of Hiroshima was essentially leveled. Following this attack, on August 9, 1945, another atomic bomb, nicknamed the "Fat Man," was dropped on Nagasaki, Japan. The same effects were felt in this city. The United States was ready to drop another atomic bomb in the third week of August, 3 more in September, and 3 more in October if necessary. However, on August 15, 1945, Japan surrendered to the Allies and World War II was officially over.
The Workings of the Atomic Bomb
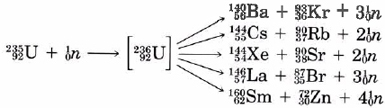
The Little Boy nuclear weapon contained enriched uranium-235. A crucial feature of this weapon was the fission of uranium. This type of fission reaction produces more neutrons than it consumes. As can be seen from Eqs. (1), for every neutron captured by a \(\ce{^{235}_{92}U}\) nucleus, between two and four neutrons are produced. Suppose now that we have a very large sample of the pure \(\ce{^{235}_{92}U}\) isotope and a stray neutron enters this sample. As soon as it hits a \(\ce{^{235}_{92}U}\) nucleus, fission will take place and about three neutrons will be produced. These in turn will cause the fission of three more 235U nuclei, producing a total of nine neutrons. A third fission cycle will produce 27 neutrons; a fourth, 81; and so on. This process (which is called a chain reaction) escalates very rapidly. Within a few microseconds, a very large number of nuclei fission reactions occur releasing a tremendous amount of energy, resulting in an atomic explosion.
 )
)
There are two reasons why a normal sample of uranium metal does not spontaneously explode in this way. First, natural uranium consists mainly of the isotope \(\ce{^{238}_{92}U}\), while the fissionable isotope \(\ce{^{235}_{92}U}\) comprises only 0.7 percent of the total. Most of the neutrons produced in a given fission process are captured by \(\ce{^{238}_{92}U}\) nuclei without any further production of neutrons. The escalation of the fission process thus becomes impossible. However, even a sample of pure 235U will not always explode spontaneously. If it is sufficiently small, many of the neutrons will escape into the surroundings without causing further fission. The sample must exceed a critical mass before an explosion results. In an atomic bomb, several pieces of fissionable material, all of which are below the critical mass, are held sufficiently far apart to prevent the chain reaction from occurring. When these are suddenly brought together, an atomic explosion results immediately.
Isotopic Enrichment
A great deal of the five years of the Manhattan Project was spent separating the 0.7% of 235U from the more abundant 238U. Most of this work was performed at the at Kiingston Demolition Range and Clinton Engineer Works in Oak Ridge, Tennessee. These two isotopes are nearly identical in physical properties, making it very challenging to isolate the uranium-235 isotope. Several methods for enriching uranium were developed and used in Oak Ridge. One method capitalized on the slight mass difference between the two isotopes. Gaseous UF6 was heated and allowed to effuse through a porous screen. Each effusion resulted in a gas that was slightly richer in the lighter isotope. Repeating this process eventually produced a compound rich enough in 235U for the purposes of bomb manufacturing.
Synthesis of Plutonium
In parallel with the work on uranium was an effort to produce plutonium-239, which was discovered at the Berkeley Radiation Laboratory in 1940. Only the first bomb dropped on Japan used uranium. The second bomb used the artificial element plutonium, produced by the neutron bombardment of 238U:
\[ \ce{^{238}_{92} U + ^{1}_0n \rightarrow ^{239}_{94}Pu + 2 ^{0}_{-1}\beta } \nonumber \]
Fission of Pu-239 occurs in much the same way as for U-235, giving a variety of products; for example,
\[ \ce{^{239}_{94} Pu + ^{1}_0n \rightarrow ^{90}_{38}Pu + ^{147}_{56}Ba + 3 ^{1}_{0}n } \nonumber \]
Again this is a highly exothermic reaction yielding about the same energy per mole (20 000 GJ mol–1) as 235U.
This immense amount of energy released is the reason why plutonium and uranium were used. By comparison, 1 ton of TNT yields about 4 GJ mol–1 of energy. This means that the atomic bombs had about 5,000 times more energy than 1 ton of TNT, explaining why the bomb was so effective and destructive.
Bomb Construction
At Los Alamos, New Mexico, J. Robert Oppenheimer, and his research group were given the task of assembling and testing the fission bomb. Both types of fuel that were manufactured (U-235 and Pu-239) would be placed separately into two different bomb designs. The smaller of the two bombs would contain U-235 and was code-named "Little Boy." The larger bomb, which housed Pu-239, was named "Fat Man." Of the two, only the Pu-239 bomb would be tested before it was dropped in wartime.
The basic design of the U-235 bomb is shown in the figure below. To prevent the spontaneous detonation of an atomic bomb, the fissile U-235 is kept in a subcritical configuration. It is then rapidly assembled into a supercritical mass using conventional explosives. Once the bomb has achieved this mass, any neutron introduced into it will be likely to initiate a chain reaction. The mechanism for "Little Boy" was a gun that fired one subcritical piece of U-235 into another to form a supercritical mass (Figure \(\PageIndex{2}\)). The pieces had to be assembled faster than the spontaneous neutrons from either U-235 or cosmic radiation. A conventional explosive in an artillery barrel could fire the U-235 mass at speeds of a few millimeters per second, fast enough to prevent a fizzle caused by a spontaneous neutron setting off a premature chain reaction.
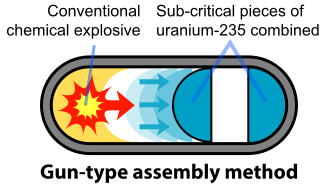
Originally, the gun-type mechanism was planned for both the U-235 and Pu-239 weapons. However, a problem arose with the Pu-239 bomb that required a different assembly mechanism because of the small amount of Pu-240 that is produced with the Pu-239 in the reactor. Pu-240 emits large numbers of neutrons spontaneously: 1,030 neutrons per gram per second compared with 0.0004 neutrons per gram per second for U-235. Even at a concentration of 1% Pu-240 in the fissile Pu-239, the required mass of Pu emits 52,000 neutrons per second or one neutron every 20 microseconds. Thus, it is very probable that a neutron from Pu-240 will initiate a premature chain reaction during the critical last 100 microseconds of the critical mass in a gun-type assembly. This problem was discovered in mid-1944, well after the start of the construction of the massive Hanford plutonium production facilities.
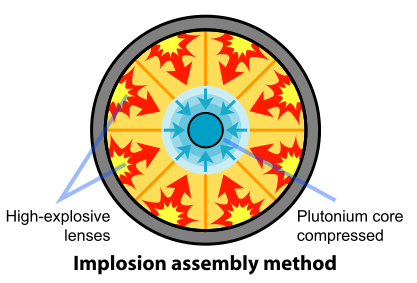
The removal of the Pu-240 was impractical. So the scientists and engineers looked for a faster method of assembling the plutonium. A mechanism based on implosion provided the solution to this problem. In this design, the fissile material is shaped into a single sphere with a mass slightly less than critical (Figure \(\PageIndex{3}\)). Layers of carefully shaped high explosives surround the sphere. When the explosives are detonated, the force of the shock wave compresses the fissile material into a smaller volume, forming a supercritical mass. This method of assembly is much faster than the gun-type mechanism and thus eliminates the problems resulting from spontaneous neutron emission of Pu-240. The spherical mass resulted in a pumpkin-shaped weapon called "Fat Man".
Watch the from the 23-minute mark through the 32-minute mark of the third installment of the History Channel's Manhattan Project and answer the questions below.
- What were the problems with the two different types of fuel that were to be used in the fission weapons?
- What was the configuration or set-up of the Pu-239 bomb?
- The combination of electromagnetic separation and gaseous diffusion enriched enough U-235 to produce how many bombs?
- Why was the U-235 bomb never tested?
- When was the construction of the U-235 completed?
- How much did Truman know about the nuclear bombs when FDR was alive?
- Who was selected to pilot the plane for the nuclear weapons?
- What island would serve as a base to house the nuclear bombs before delivering them to their targets?
In July 1945, the United States had enough fissile material for one uranium and two plutonium weapons. The scientists and engineers felt confident that the gun-type assembly mechanism for Little Boy would function properly. Besides, they did not have the material for a test device. They were less confident about the implosion mechanism on the plutonium weapon and felt that a test was necessary. On July 16, 1945, the first nuclear device, known as "The Gadget", was placed on a 100-foot tower and successfully detonated in the Alamogordo Desert, 200 miles south of Los Alamos (Figure \(\PageIndex{4}\)).
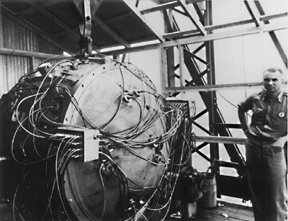
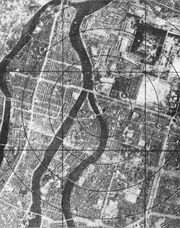
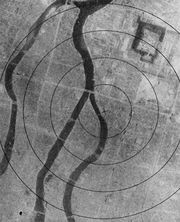
As the war with Japan continued and a costly allied invasion loomed as a real possibility, President Truman approved the use of nuclear weapons against selected Japanese targets. The U.S. Army Air Force received orders to use these weapons anytime after August 3, 1945. On August 6, "Little Boy" was dropped on Hiroshima (Figure \(\PageIndex{5}\)).

Little Boy was a uranium weapon containing 141.4 pounds of fissionable material containing 82.7% U-235. Only about two pounds fissioned, releasing an energy equivalent to 15-16,000 tons of TNT. The immediate effects of the blast killed an estimated 70,000 people, and by the end of 1945 an additional 20,000 to 70,000 deaths occurred, many due to lack of adequate medical resources.
Three days later, "Fat Man" destroyed a large part of Nagasaki (Figure \(\PageIndex{6}\)). Fat Man contained 13.6 pounds of Pu-239, of which only 2 pounds underwent fission. The explosive yield was equivalent to about 22,000 tons of TNT. Fat man resulted in 35,000 immediate deaths. By the end of 1945, at least 70,000 people perished in this event.

Figure \(\PageIndex{6}\): Fat Man – One of many Pu-239 nuclear weapons constructed during WWII. Courtesy of the U.S. Department of Defense
Start the video below at the 23:20 mark and stop it at the 29-minute setting. Then, answer the questions below:
- When and where was the first nuclear bomb tested?
- Was the bomb placed on the ground?
- What were scientists afraid the first nuclear bomb would do?
- What did Edward Teller (father of the fusion bomb) apply to his skin before the bomb was ignited? Why did he do this?
- What did the bomb do to the tower and the surrounding sand?
- In the photograph, where did General Grove focus his attention?
Nuclear Fallout
Nuclear fallout refers to the radioactive material that "falls out" of the atmosphere after a nuclear explosion (reactor or bomb). It consists of dust and radioactive particles that can contaminate an area with radioactivity and pose a huge health hazard to biological organisms. It can contaminate the animal food chain which can have drastic effects on the affected region. Weather has a huge impact on fallout, wind currents can spread radioactive fallout either over a large area, such as in the case of Castle Bravo, or not.

In regards to nuclear reactions, fission produces more fallout products that fusion. Intense heat of the latter type of reactions reduces the amount and type of radioisotopes produced. Although more powerful, fusion is a "cleaner" type of nuclear reaction. By increasing the fission component of a fusion weapon, bombs can be designed to generate more fallout products.Of the different types of isotopes that fission produces, the most worrisome are Sr-90, Cs-137,and I-131. The first of these isotopes has a half-life of 28.8 years and decays by gamma and beta emission. Once ingested or inhaled, Sr-90 deposits in teeth, bone,and bone marrow. Sr-90 exposure can lead to bone cancer and/or leukemia. Cs-137 (t1/2 = 30.17 years) emits beta and gamma radiation. This ionizing radiation deposits in soft tissues of the body. In addition, this particular radioisotope is water soluble and can easily contaminant food and water supplies. I-131 had a half-life over 8.1 days and undergoes gamma/beta decay. Once this fallout product enters the body, it attacks the thyroid gland. Extensive damage could affect heart rate, blood pressure, body temperature, and childhood growth. In addition, I-131 exposure could result in thyroid cancer.
Watch the fallout video and then answer the questions below:
Summary
- In an atomic bomb, several pieces of fissionable material are held sufficiently far apart so no chain reaction can occur. When these components are brought together, the critical mass is reached and an atomic explosion results immediately.
- The two fission bombs assembled at Los Alamos, New Mexico, by J. Robert Oppenheimer, and his research group were manufactured with two types of fuel namely, U-235 and Pu-239.
- The smaller of the two bombs contained U-235 and was code-named "Little Boy." This bomb was dropped in Hiroshima, Japan on Aug.6, 1945.
- The larger bomb, which housed Pu-239, was named "Fat Man." This bomb was dropped in Nagasaki, Japan on Aug.9, 1945.
- Nuclear fallout refers to the radioactive material that "falls out" of the atmosphere after a nuclear explosion (reactor or bomb).
Contributors and Attributions
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).
-
Ed Vitz (Kutztown University), John W. Moore (UW-Madison), Justin Shorb (Hope College), Xavier Prat-Resina (University of Minnesota Rochester), Tim Wendorff, and Adam Hahn.
Frank A. Settle (Washington and Lee University)
- Muneeba Ali (Furman University)

