4.4: Acid Rain and Water Hardness
- Page ID
- 466595
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain how combustion processes affect water sources.
- Define the pH and the composition of acid rain (three specific formulas/names).
- Know the precursors of acid rain.
- Describe the environmental and biological effects of acid rain.
- Understand the differences between hard and soft water.
The US Safe Drinking Water Act defines the term "contaminant" as meaning any physical, chemical, biological, or radiological substance or matter in water. Therefore, the "contaminant" definition broadly applies to anything other than water molecules that are present. More information can be found at the EPA Drinking Water Contaminant Website. Drinking water may reasonably be expected to contain at least small amounts of some contaminants. Some drinking water contaminants may be harmful if consumed at certain levels in drinking water while others may be harmless. The presence of contaminants does not necessarily indicate that the water poses a health risk.
Air Pollutants
Water can become contaminated at any part of the water cycle. Air pollution can affect water vapor and liquid water. Combustion sources like vehicles and power plants generate compounds like \(\ce{NO_xs}\), \(\ce{SO_xs}\), \(\ce{CO}\), \(\ce{CO2}\), and other various inorganic and organic volatile organic compounds (VOC) species. Some of these compounds can become soluble in water. This could affect pH (or acidity) level of water surface water. Rainwater with a pH below 5.6 is considered to be acid rain.
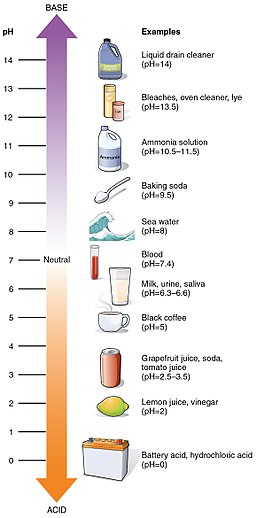
Normally, water is neither acidic or basic, and has a neutral pH (pH= 7). When \(\ce{NO_xs}\), \(\ce{SO_xs}\), \(\ce{CO}\), \(\ce{CO2}\) enter the water cycle, then the pH level is lowered below 7.0. If these gases are absorbed in rain clouds, then acid rain results. Specific acids involved in acid rain are sulfuric acid (H2SO4), nitric acid (HNO3), and carbonic acid (H2CO3). This environmental problem affects living organisms and building materials. Acid solutions can corrode metals and make them soluble as well.
ADAPT \(\PageIndex{1}\)
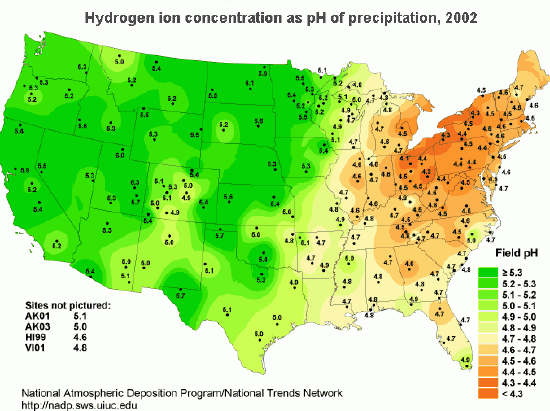
The acidity of rain is measured by collecting samples of rainwater and measuring its pH. To find the distribution of acid rain, weather conditions are monitored and rain samples collected at sites all over the country. The areas of greatest acidity (lowest pH values) are located in the Northeastern United States. This pattern of high acidity is caused by a large number of cities, the dense population, and the concentration of power and industrial plants in the Northeast. In addition, the prevailing wind direction brings storms and pollution to the Northeast from the Midwest, and dust from the soil and rocks in the Northeastern United States is less likely to neutralize acidity in the rain.
ADAPT \(\PageIndex{2}\)
However, the majority of acid rain is accounted for by the presence of sulfuric acid (\(\ce{H2SO4}\)).
\[ \ce{SO2(g) + O2(g) -> SO3(g)} \nonumber \]
Sulfur dioxide reacts with oxygen to form sulfur trioxide. Sulfur trioxide can then react with water to form sulfuric acid
\[\ce{SO3(g) + H2O -> H2SO4} \label{eq10} \]
Although sulfuric acid may be produced naturally in small quantities from biological decay and volcanic activity, almost all sulfur dioxide, that is subsequently converted to sulfuric acid, is produced by human activity, especially from the combustion of sulfur-containing fossil fuels in power plants.
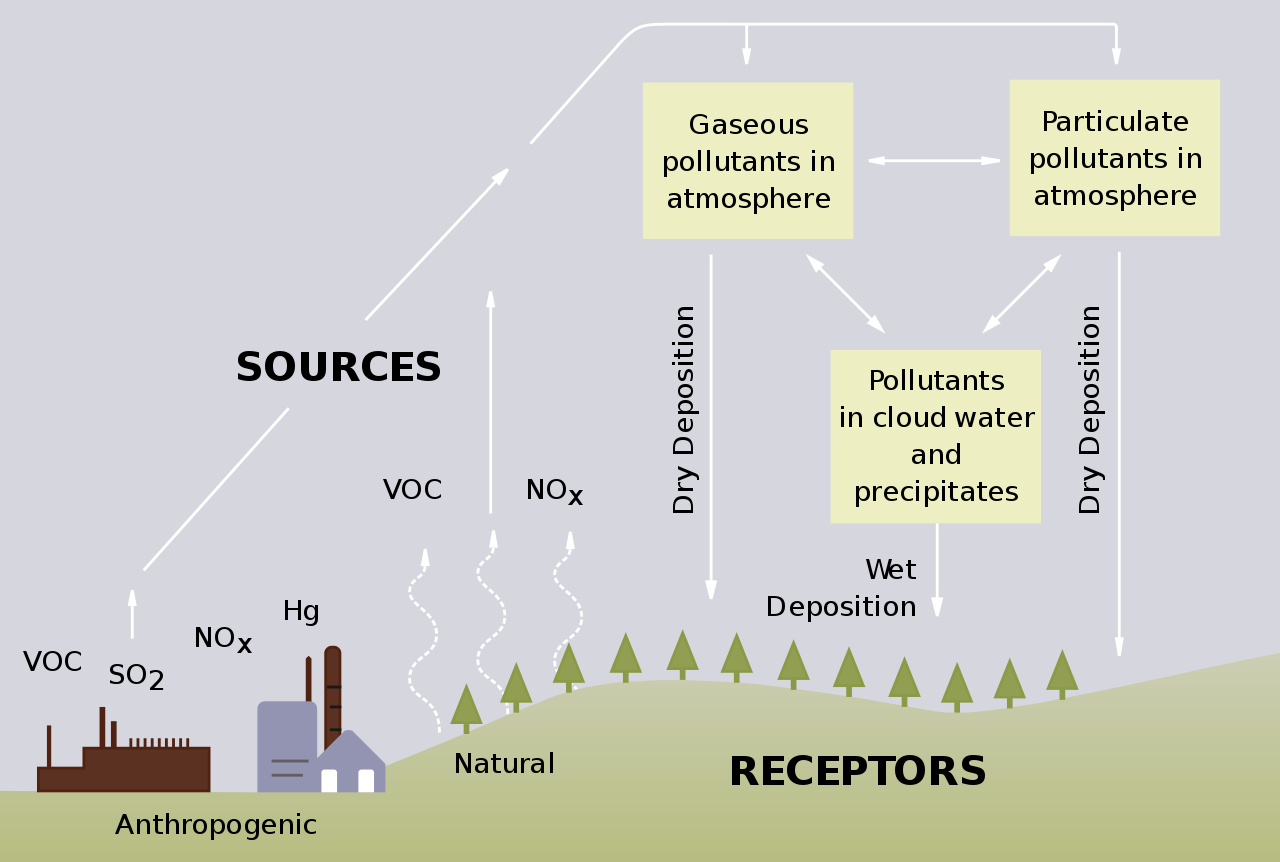
Some acid rain is accounted for by nitric acid (\(\ce{HNO3}\)). This compound can originate from natural processes but is primarily released from high-temperature air combustion, such as the reactions in car engines and power plants. These processes produce large amounts of \(\ce{NO}\) gas. This gas then forms nitric acid via several steps. Nitric oxide (\(\ce{NO}\)) can also be formed during lightning storms by the reaction of nitrogen and oxygen
\[ \ce{N2 (g) + O2(g) -> 2NO(g)} \label{eq1} \]
and \(\ce{NO}\) is oxidized to nitrogen dioxide (\(\ce{NO2}\))
\[ \ce{NO(g) + 1/2 O2(g) -> NO2 (g)} \label{eq2} \]
\(\ce{NO2(g)}\) then reacts with water droplets to generate nitric acid (\(\ce{HNO3}\))
\[\ce{3NO2(g) + H2O(l) -> 2HNO3(aq) + NO(g)} \nonumber \]
| Combustion products | Sources |
|---|---|
| CO2 and CO | Combustion of any material (any fuel or tree) |
| NOX (NO2 and NO3) | High-temperature combustion of any fuel ( gas, diesel, or coal), a product of lightning |
| SOx (SO2 and SO3) | Combustion of sulfur-based fuels (diesel and coal), volcanic release |
| VOC (volatile organic compound) | Combustion of any carbon-based fuel (gas, diesel, or coal), fumes from paints or solvents |
ADAPT \(\PageIndex{3}\)
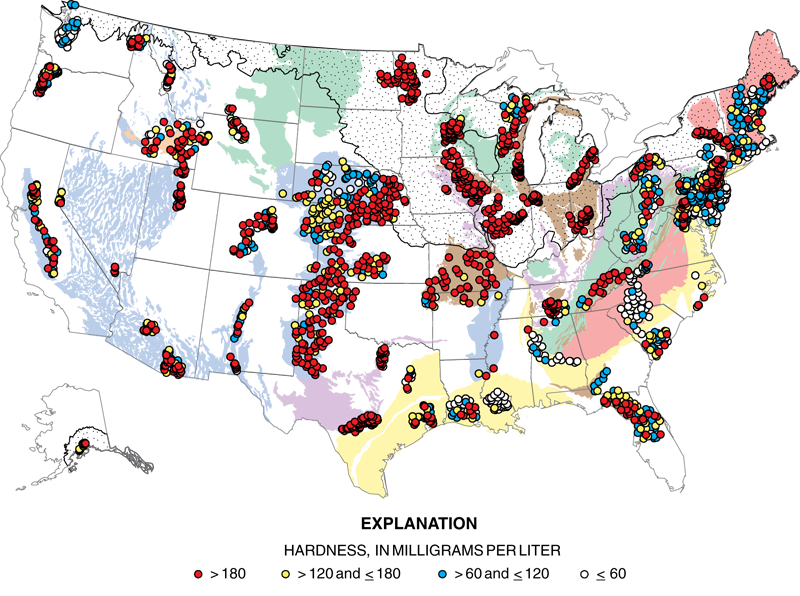
Hard Water
When water is described as being hard, this means it has a large concentration of minerals (this typically means metal ions). The ions that contribute to this problem are calcium (Ca2+), magnesium (Mg2+), carbonate (CO3)2-, and iron (Fe2+/Fe3+). Hard water is not toxic, but can affect industrial processes. These ions will bond together with nonmetal ions or polyatomic anions to form insoluble salts that are called precipitates. The buildup of these newly formed solids is called scale. Precipitates can collect in boilers, cooling towers, and any equipment that employs water. This buildup can affect how the equipment works by damaging it internally.
Watch this video below to get an idea of what hard water is.
- What chemical species cause hard water?
- How does hard water affect industry?
- Hard water is known to contribute to the formation of kidney stones. How else can this type of water affect the body?
Soft Water
Soft water does not contain an excessive amount of alkaline minerals. Instead, potassium (K+) and sodium (Na+) ions are present. Soft water does not form scale but does produce excessive lathers with soap. Although industry and consumers prefer soft water, the sodium content can negatively affect human health if ingested at a high concentrations.
ADAPT \(\PageIndex{4}\)
ADAPT \(\PageIndex{5}\)

