2.8: Molar Mass to Mole Conversions
- Page ID
- 466584
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Perform conversions between mass and moles of a substance.
- Convert from mass or moles of one substance to mass or moles of another substance in a chemical reaction.
- Use a balanced chemical equation to determine molar relationships between substances.
Molar Mass
The molar mass of any substance is the mass in grams of one mole of representative particles of that substance. The representative particles can be atoms, molecules, or formula units of ionic compounds. This relationship is frequently used in the laboratory. To calculate the molar mass of a substance, add the atomic weight of every atom found in that substance. For example, water (or H2O) consists of two atoms of hydrogen and 1 atom of oxygen. The molar mass equals the sum of the atomic weight of each atom in the molecule (1.008 amu + 1.008 amu + 15.999 amu). For water, the molar mass is equal to 18.015 amu (or g/mol).
ADAPT \(\PageIndex{1}\)
ADAPT \(\PageIndex{2}\)
The simplest type of manipulation using molar mass as a conversion factor is a mole-mass conversion (or its reverse, a mass-mole conversion). In such a conversion, we use the molar mass of a substance as a conversion factor to convert mole units into mass units (or, conversely, mass units into mole units).
When considering the element, aluminum, we can use the periodic table of elements to look up the atomic weight of this element (26.98 amu). This value also means that 1 mol of Al has a mass of 26.98 g. Stated mathematically,
1 mol Al = 26.98 g Al
We can divide both sides of this expression by either side to get one of two possible conversion factors:
\[\mathrm{\dfrac{1\: mol\: Al}{26.98\: g\: Al}\, and\, \dfrac{26.98\: g\: Al}{1\: mol\: Al}} \label{Eq1} \]
The first conversion factor can be used to convert from mass to moles, and the second converts from moles to mass. Both can be used to solve problems that would be hard to do “by eye.”
What is the mass of 3.987 mol of Al?
Solution
| Steps for Problem Solving | Example |
|---|---|
| Identify the "given" information and what the problem is asking you to "find." | Given: 3.987 mol of Al Find: g Al |
| List other known quantities | 1 mol Al = 26.98 g Al |
| Prepare a concept map and use the proper conversion factor. | 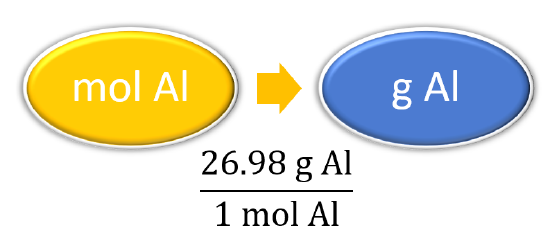 |
| Cancel units and calculate. | \(3.987 \: \cancel{\text{mol} \: \ce{Al}} \times \dfrac{26.98 \: \text{g} \: \ce{Al}}{1 \: \cancel{\text{mol} \: \ce{Al}}} = 107.6 \: \text{g} \: \ce{Al}\) |
| Think about your result. | The calculated value makes sense because it is almost four times times the mass for 1 mole of aluminum. Our final answer is expressed to four significant figures. |
How many moles are present in 100. g of Al? (Hint: you will have to use the other conversion factor we obtained for aluminum.)
Answer
3.71 g Al
Conversions like this are possible for any substance, as long as the proper atomic mass, formula mass, or molar mass is known (or can be determined) and expressed in grams per mole. Figure \(\PageIndex{1}\) is a chart for determining what conversion factor is needed, and Figure \(\PageIndex{2}\) is a flow diagram for the steps needed to perform a conversion.
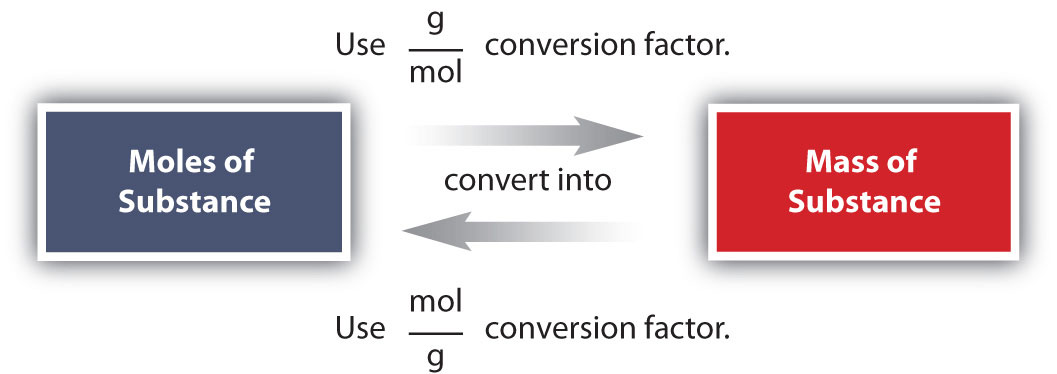
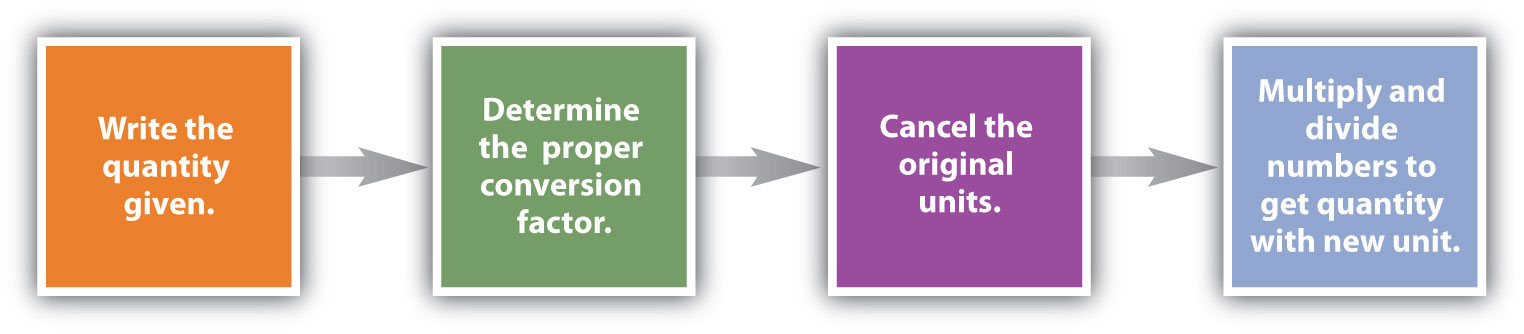
Suppose that for a certain experiment you need 3.00 moles of calcium chloride \(\left( \ce{CaCl_2} \right)\). Since calcium chloride is a solid, it would be convenient to use a balance to measure the mass that is needed. Dimensional analysis will allow you to calculate the mass of \(\ce{CaCl_2}\) that you should measure as show in Example \(\PageIndex{3}\).
Calculate the mass of 3.00 moles of calcium chloride (CaCl2).

Solution
| Steps for Problem Solving | Example |
|---|---|
| Identify the "given" information and what the problem is asking you to "find." | Given: 3.00 moles of CaCl2 Find: g CaCl2 |
|
List other known quantities (molar mass is calculated by adding the atomic weight of each atom in the molecule) |
1 ml CaCl2 = 110.98 g CaCl2 |
| Prepare a concept map and use the proper conversion factor. | |
| Cancel units and calculate. | \(3.00 \: \cancel{\text{mol} \: \ce{CaCl_2}} \times \dfrac{110.98 \: \text{g} \: \ce{CaCl_2}}{1 \: \cancel{\text{mol} \: \ce{CaCl_2}}} = 333 \: \text{g} \: \ce{CaCl_2}\) |
| Think about your result. |
What is the mass of \(7.50 \: \text{mol}\) of (calcium oxide) \(\ce{CaO}\)?
- Answer:
- 420.60 g
How many moles are present in 108 grams of water?
Solution
| Steps for Problem Solving | Example |
|---|---|
| Identify the "given" information and what the problem is asking you to "find." | Given: 108 g H2O Find: mol H2O |
|
List other known quantities (molar mass is calculated by adding the atomic weight of each atom of the molecule or compound) |
\(1 \: \text{mol} \: \ce{H_2O} = 18.02 \: \text{g}\) H2O |
| Prepare a concept map and use the proper conversion factor. | |
| Cancel units and calculate. | \(108 \: \cancel{\text{g} \: \ce{H_2O}} \times \dfrac{1 \: \text{mol} \: \ce{H_2O}}{18.02 \: \cancel{\text{g} \: \ce{H_2O}}} = 5.99 \: \text{mol} \: \ce{H_2O}\) |
| Think about your result. |
What is the mass of \(7.50 \: \text{mol}\) of Nitrogen gas \(\ce{N2}\)?
- Answer:
- 210 g
ADAPT \(\PageIndex{3}\)
ADAPT \(\PageIndex{4}\)
Summary
- The molar mass of an atom, molecule, or compound can be used as a conversion factor to convert from grams to moles or from moles to grams.
Contributors and Attributions
- TextMap: The Basics of GOB Chemistry (Ball et al.)
Henry Agnew (UC Davis)
- Erin Avram (Cleveland State University)

