3.8: Prostaglandins and other Eicosanoids
- Page ID
- 233996
Learning Objectives
After completing this section, you should be able to
- describe the general structure of the prostaglandins, and identify a prostaglandin from a given list of organic structures.
- identify at least two important biological functions of prostaglandins.
Key Terms
Make certain that you can define, and use in context, the key term below.
- prostaglandin
Prostaglandin Structure
Prostaglandins are chemical messengers synthesized in the cells in which their physiological activity is expressed. They are unsaturated fatty acids containing 20 carbon atoms and are synthesized from arachidonic acid—a polyunsaturated fatty acid—when needed by a particular cell. They are called prostaglandins because they were originally isolated from semen found in the prostate gland. The unique shape of the arachidonic acid caused by a series of cis double bonds helps to put it into position to make the five member ring of the prostaglandin.
Prostaglandins are one example of biologically important class of fatty acids called eicosanoids. Derived primarily from arachidonic acid (5,8,11,14-eicosatetraenoic acid), eicosanoids include prostaglandins, leukotrienes, and thromboxanes. It is now known that they are synthesized in nearly all mammalian tissues and affect almost all organs in the body. The five major classes of prostaglandins are designated as PGA, PGB, PGE, PGF, and PGI. Subscripts are attached at the end of these abbreviations to denote the number of double bonds outside the five-carbon ring in a given prostaglandin.
The metabolic pathways by which arachidonic acid is converted to the various prostaglandins and eicosanoids are complex and will not be discussed here. A rough outline of some of the transformations that take place is provided below. It is helpful to view arachadonic acid in the coiled conformation shown in the shaded box.
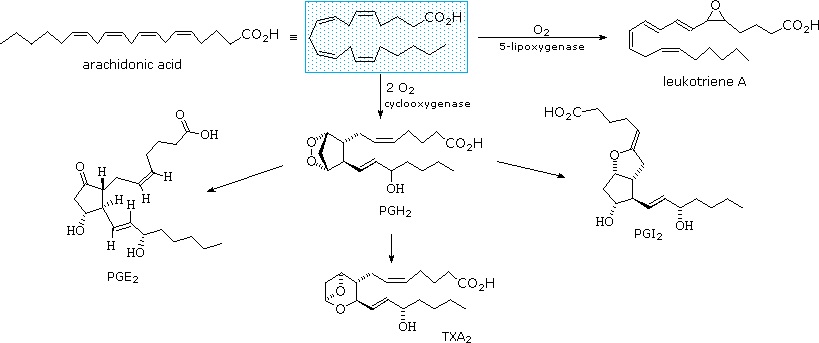
Functions of Prostaglandins
Prostaglandins are chemical messengers (hormones) synthesized in the cells in which their physiological activity is expressed. The prostaglandins are among the most potent biological substances known. Slight structural differences give them highly distinct biological effects; however, all prostaglandins exhibit some ability to induce smooth muscle contraction, lower blood pressure, and contribute to the inflammatory response. Aspirin and other nonsteroidal anti-inflammatory agents, such as ibuprofen, obstruct the synthesis of prostaglandins by inhibiting cyclooxygenase, the enzyme needed for the initial step in the conversion of arachidonic acid to prostaglandins.
There are a variety of physiological effects for prostaglandins, including:
- Activation of the inflammatory response, production of pain, and fever. When tissues are damaged, white blood cells flood to the site to try to minimize tissue destruction. Prostaglandins are produced as a result.
- Blood clots form when a blood vessel is damaged. A type of prostaglandin called thromboxane stimulates constriction and clotting of platelets. Conversely, PGI2, is produced to have the opposite effect on the walls of blood vessels where clots should not be forming.
- Certain prostaglandins are involved with the induction of labor and other reproductive processes. PGE2 causes uterine contractions and has been used to induce labor.
- Prostaglandins are involved in several other organs such as the gastrointestinal tract (inhibit acid synthesis and increase secretion of protective mucus), increase blood flow in kidneys, and leukotriens promote constriction of bronchi associated with asthma.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
- Charles Ophardt, Professor Emeritus, Elmhurst College; Virtual Chembook
- Fatty Acids. (2020, August 17). Retrieved May 11, 2021, from https://chem.libretexts.org/@go/page/16123. " Fatty Acids" by LibreTexts is licensed under CC BY-NC-SA .

