4.7: Factors Affecting the SN1 Reaction
- Page ID
- 227561
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objective
- determine the rate law & predict the mechanism based on its rate equation or reaction data for SN1 reactions
- predict the products and specify the reagents for SN1 reactions with stereochemistry
- propose mechanisms for SN1 reactions
- draw and interpret Reaction Energy Diagrams for SN1 reactions
In order of decreasing importance, the factors impacting SN1 reaction pathways are
- structure of the alkyl halide
- stability of the leaving group
- type of solvent.
The unimolecular transition state of the SN1 pathway means that structure of the alkyl halide and stability of the leaving group are the primary considerations. Alkyl halides that can ionize to form stable carbocations are more reactive via the SN1 mechanism. Because carbocation stability is the primary energetic consideration, stabilization of the carbocation via solvation is also an important consideration.
Alkyl Halide Structure
Alkyl halides that can ionize to form stable carbocations are more reactive via the SN1 mechanism. The stability order for carbocation is as follows:
Carbocation stability order: 3º>2º>1º>methyl. Image by Alatleephillips, CC BY-SA 4.0, via Wikimedia Commons
That order means that a tertiary alkyl halide is more reactive towards SN1 compared to secondary and primary alkyl halides respective. Methyl halides almost never react via an SN1 mechanism. Notice that this reactivity order is the exact opposite of SN2 reactions.
Effects of Leaving Group
An SN1 reaction also speeds up with a good leaving group. This is because the leaving group is involved in the rate-determining step. A good leaving group wants to leave so it breaks the C-Leaving Group bond faster. Once the bond breaks, the carbocation is formed and the faster the carbocation is formed, the faster the nucleophile can come in and the faster the reaction will be completed.
A good leaving group is a weak base because weak bases can hold the charge. They're happy to leave with both electrons and in order for the leaving group to leave, it needs to be able to accept electrons. Strong bases, on the other hand, donate electrons which is why they can't be good leaving groups. As you go from left to right on the periodic table, electron donating ability decreases and thus ability to be a good leaving group increases. Halides are an example of a good leaving group whos leaving-group ability increases as you go down the column.
.jpg?revision=1&size=bestfit&width=300&height=74)
Solvent Effects on the SN1 Reaction
To facilitate the formation of ions, a polar solvent is needed. In the case of SN1 eactions, polar protic solvents speed up the rate of SN1 reactions because the polar solvent helps stabilize the transition state and carbocation intermediate. Since the carbocation is unstable, anything that can stabilize this even a little will speed up the reaction. Polar aprotic solvents have a dipole moment, but their hydrogen is not highly polarized.
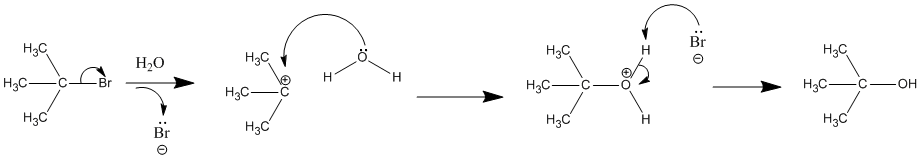
Effects of Nucleophile
The strength of the nucleophile does not affect the reaction rate of SN1 because the nucleophile is not involved in the rate-determining step. Therefore, weak nucleophiles tend to favor SN1 mechanism. Typical SN1 reactions take place where the solvent is the nucleophile. Examples: H2O, alcohols (ROH), CH3CN, etc.
Exercises
1. Rank the following by increasing reactivity in an SN1 reaction.
2. 3-bromo-1-pentene and 1-bromo-2-pentene undergo SN1 reaction at almost the same rate, but one is a secondary halide while the other is a primary halide. Explain why this is.
3. Label the following reactions as most likely occuring by an SN1 or SN2 mechanism. Suggest why.
- Answers
-
1. Consider the stability of the intermediate, the carbocation.
A < D < B < C (most reactive)
2. They have the same intermediates when you look at the resonance forms.
3. A – SN1 *poor leaving group, protic solvent, secondary cation intermediate
B – SN2 *good leaving group, polar solvent, primary position.
Citations and attributions
Characteristics of the Sₙ1 Reaction. (2020, May 30). Retrieved May 23, 2021, from https://chem.libretexts.org/@go/page/45179


