4.1: Alkyl Halides - Structure and Physical Properties
- Page ID
- 227554
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objective
- classify alkyl halides
- predict relative boiling points and solubility of alkyl halides
Introduction
Alkyl halides are also known as haloalkanes. Alkyl halides are compounds in which one or more hydrogen atoms in an alkane have been replaced by halogen atoms (fluorine, chlorine, bromine or iodine). We will only look at compounds containing one halogen atom like th compounds below.
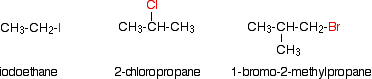
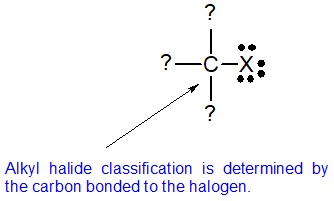
Alkyl halides fall into different classes depending on how the halogen atom is positioned on the chain of carbon atoms. Alkyl halides can be classified as primary, secondary, or tertiary. The chemical reactivity of alkyl halides is frequently discussed using alkyl halide classifications to help discern patterns and trends. Because the neutral bonding pattern for halogens is one bond and three lone pairs, the carbon and halogen always share a single bond. Alkyl halide classification is determined by the bonding pattern of the carbon atom bonded to the halogen as shown in the diagram below.
Primary alkyl halides
In a primary (1°) haloalkane, the carbon bonded to the halogen atom is only attached to one other alkyl group. Some examples of primary alkyl halides include thecompounds below.
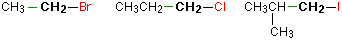
Notice that it doesn't matter how complicated the attached alkyl group is. In each case there is only one linkage to an alkyl group from the CH2 group holding the halogen. There is an exception to this: CH3Br and the other methyl halides are often counted as primary alkyl halides even though there are no alkyl groups attached to the carbon with the halogen on it.
Secondary alkyl halides
In a secondary (2°) haloalkane, the carbon bonded with the halogen atom is joined directly to two other alkyl groups that can be the same or different. Some examples of secondary alkyl halides include thecompounds below.
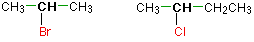
Tertiary alkyl halides
In a tertiary (3°) halogenoalkane, the carbon atom holding the halogen is attached directly to three alkyl groups, which may be any combination of same or different. Some examples of tertiary alkyl halides include thecompounds below.
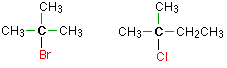
Nomenclature
Many organic compounds are closely related to the alkanes and this similarity is incorporated into many common names. The reactions of alkanes with halogens produce halogenated hydrocarbons, compounds in which one or more hydrogen atoms of a hydrocarbon have been replaced by halogen atoms: The replacement of only one hydrogen atom gives an alkyl halide (or haloalkane). The common names of alkyl halides consist of two parts: the name of the alkyl group plus the stem of the name of the halogen, with the ending -ide. In the IUPAC system, alkyl halides are named as substituted alkanes.
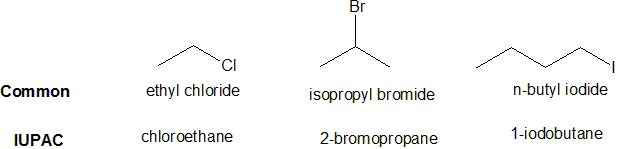
Examples
Give the common and IUPAC names for each compound.
- CH3CH2CH2Br
- (CH3)2CHCl
- Give the IUPAC name for each compound.
a)
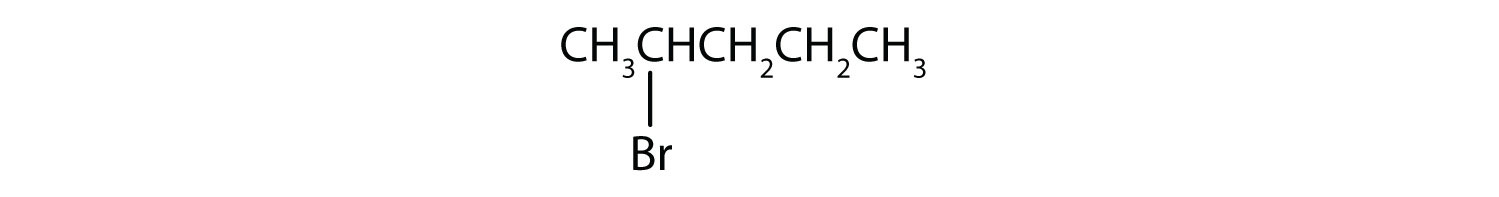 b)
b)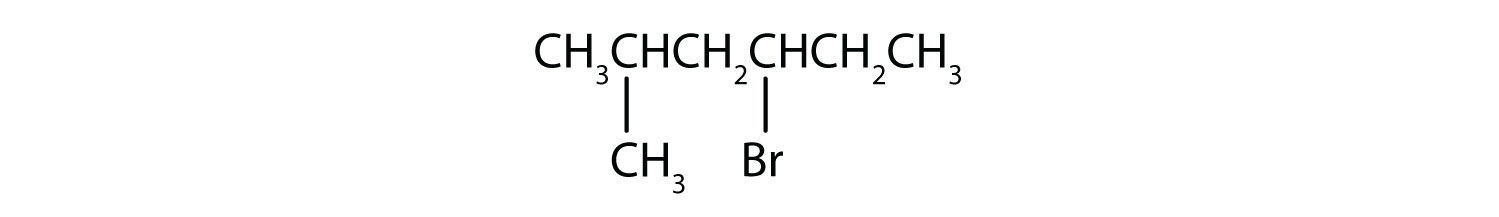
SolutionS
- The alkyl group (CH3CH2CH2–) is a propyl group, and the halogen is bromine (Br). The common name is therefore propyl bromide. For the IUPAC name, the prefix for bromine (bromo) is combined with the name for a three-carbon chain (propane), preceded by a number identifying the carbon atom to which the Br atom is attached, so the IUPAC name is 1-bromopropane.
- The alkyl group [(CH3)2CH–] has three carbon atoms, with a chlorine (Cl) atom attached to the middle carbon atom. The alkyl group is therefore isopropyl, and the common name of the compound is isopropyl chloride. For the IUPAC name, the Cl atom (prefix chloro-) attached to the middle (second) carbon atom of a propane chain results in 2-chloropropane.
- a) The parent alkane has five carbon atoms in the longest continuous chain; it is pentane. A bromo (Br) group is attached to the second carbon atom of the chain. The IUPAC name is 2-bromopentane.
b) The parent alkane is hexane. Methyl (CH3) and bromo (Br) groups are attached to the second and fourth carbon atoms, respectively. Listing the substituents in alphabetical order gives the name 4-bromo-2-methylhexane.
Exercise
1. Give common and IUPAC names for each compound.
- CH3CH2I
- CH3CH2CH2CH2F
2. Give the IUPAC name for each compound.
a)
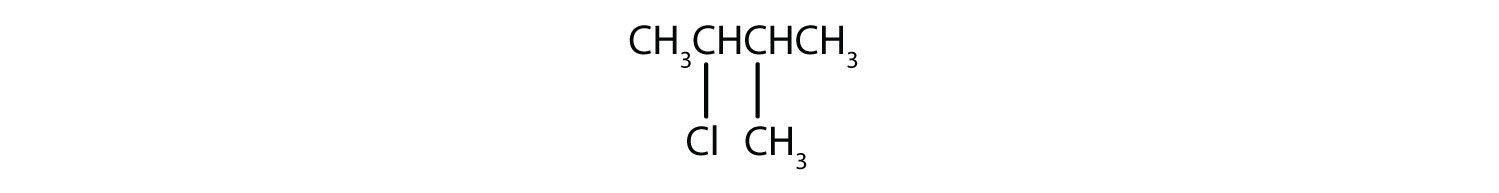
b)
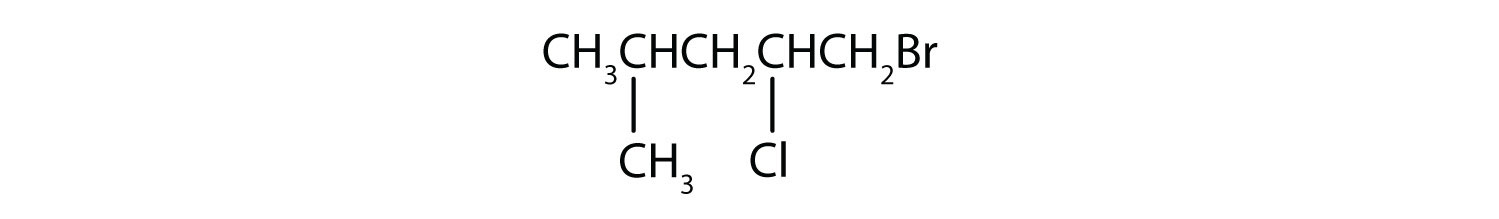
- Answer
-
1. a) ethyl iodide and iodoethane, respectively; Note the IUPAC name does not need a locator number because there is only one possible structure with two carbons and one iodine.
b) butyl fluoride and 1-fluorobutane
2. a) 2-chloro-2-methylbutane
b) 1-bromo-2-chloro-4-methylpentane
Halogens and the Character of the Carbon-Halogen Bond
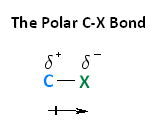
With respect to electronegativity, halogens are more electronegative than carbons. This results in a carbon-halogen bond that is polarized. As shown in the image below, carbon atom has a partial positive charge, while the halogen has a partial negative charge.
The following image shows the relationship between the halogens and electronegativity. Notice, as we move up the periodic table from iodine to fluorine, electronegativity increases.
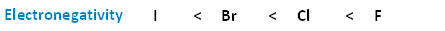
The following image shows the relationships between bond length, bond strength, and molecular size. As we progress down the periodic table from fluorine to iodine, molecular size increases. As a result, we also see an increase in bond length. Conversely, as molecular size increases and we get longer bonds, the strength of those bonds decreases.

Haloalkanes Have Higher Boiling Points than Alkanes
When comparing alkanes and haloalkanes, we will see that haloalkanes have higher boiling points than alkanes containing the same number of carbons. London dispersion forces are the first of two types of forces that contribute to this physical property. You might recall from general chemistry that London dispersion forces increase with molecular surface area. In comparing haloalkanes with alkanes, haloalkanes exhibit an increase in surface area due to the substitution of a halogen for hydrogen. The incease in surface area leads to an increase in London dispersion forces, which then results in a higher boiling point.
Dipole-dipole interaction is the second type of force that contributes to a higher boiling point. As you may recall, this type of interaction is a coulombic attraction between the partial positive and partial negative charges that exist between carbon-halogen bonds on separate haloalkane molecules. Similar to London dispersion forces, dipole-dipole interactions establish a higher boiling point for haloalkanes in comparison to alkanes with the same number of carbons.

The table below illustrates how boiling points are affected by some of these properties. Notice that the boiling point increases when hydrogen is replaced by a halogen, a consequence of the increase in molecular size, as well as an increase in both London dispersion forces and dipole-dipole attractions. The boiling point also increases as a result of increasing the size of the halogen, as well as increasing the size of the carbon chain.
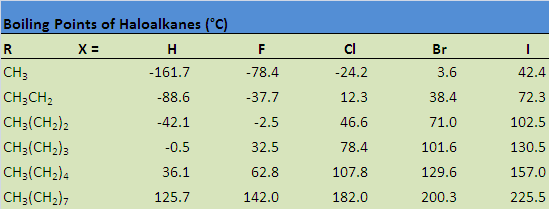
Solubility
Solubility in water
Alkyl halides have little to no solubility in water in spite of the polar carbon-halogen bond. The attraction between the alkyl halide molecules is stronger than the attraction between the alkyl halide and water. Alkyl halides have little to no solubility in water, but be aware of densities. Polyhalogenated alkanes such as dichloromethane can have densities greater than water.
Solubility in organic solvents
Alkyl halides are soluble in most organic solvents. The London Dispersion forces play a dominant role in solubility.
Exercises
Exercise
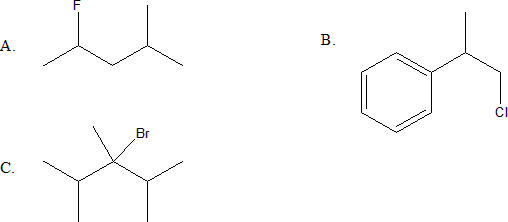
3. Classify (primary, secondary or tertiary) and give the IUPAC name for the following organohalides:
4. Classify each alkyl halide as primary, secondary, or tertiary:
a) 1-Chloro-3,3-dimethylpentane
b) 1-chloro-4-isopropylcyclohexane
c) 3-bromo-3-ethylhexane
5. Predict the solvent with great alkyl halide solubility.
-
a) water or hexane
b) water or 1-octanol
c) water or benzene
d) water or acetone
- Solutions
-
3.
a) secondary; 2-fluoro-4-methylpentane
b) primary; 1-chloro-2-methyl-2-phenylethane
c) tertiary, 3-bromo-2,3,4-trimethylpentane
4. (A) primary; (B) secondary (C) tertiary
5. a) hexane
b) benzene
c) 1-octanol
d) acetone
Alkyl halides have little to no solubility in water, but be aware of densities. Polyhalogenated alkanes can have densities greater than water.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
Jim Clark (Chemguide.co.uk)
- Rachael Curtis (UC Davis)

