Study Notes
You should be careful to remember that iodine and fluorine cannot be introduced into an aromatic ring by the method used for bromine and chlorine. On its own, iodine is unreactive with aromatic rings, but one method for aromatic iodination is treatment in the presence of a copper salt such as copper(II)chloride where I2 is oxidized to the more electrophilic species I+.
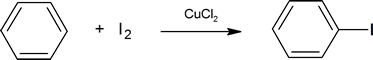
In contrast, fluorine is too reactive, so it cannot be used directly for aromatic flourination. However, fluorinating agents like 1-(chloromethyl)-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane ditetrafluoroborate (also known as F-TEDA-BF4) sold commercially as Sectfluor® offer convenient sources of “F+” for this type of reaction.
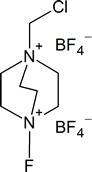
F-TEDA-BF4
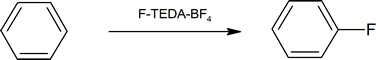
The overall equation for the formation of nitronium ions by the action of sulfuric acid on nitric acid is
$\ce{\sf{HNO3 + 2H2SO4 <=> H3O+ + NO2+ + HSO4- }}$
The ability of compounds such as nitronium tetrafluoroborate to bring about the nitration of aromatic compounds is good evidence in support of the proposed mechanism.
The nitration of an aromatic ring is an important synthetic pathway to generating arylamines. The reaction below shows one common method of reducing the nitro group. (Amines are examined in more detail in Chapter 24.)
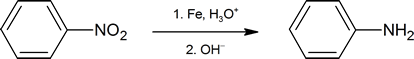
Halogenation of Benzene
Halogenation is an example of electrophillic aromatic substitution. In electrophilic aromatic substitutions, a benzene is attacked by an electrophile which results in substition of hydrogens. However, halogens are not electrophillic enough to break the aromaticity of benzenes, which require a catalyst to activate.
Activation of Halogen
(where X= Br or Cl, we will discuss further in detail later why other members of the halogen family Flourine and Iodine are not used in halogenation of benzenes)
.jpg?revision=1&size=bestfit&width=272&height=170)
Hence, Halogen needs the help and aid of Lewis Acidic Catalysts to activate it to become a very strong electrophile. Examples of these activated halogens are Ferric Hallides (FeX3) Aluminum Halides (AlX3) where X= Br or Cl. In the following examples, the halogen we will look at is Bromine.
In the example of bromine, in order to make bromine electrophillic enough to react with benzene, we use the aid of an aluminum halide such as aluminum bromide.
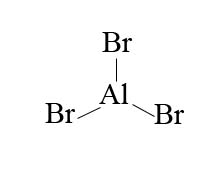
With aluminum bromide as a Lewis acid, we can mix Br2 with AlBr3 to give us Br+. The presence of Br+ is a much better electrophile than Br2 alone. Bromination is acheived with the help of AlBr3 (Lewis acid catalysts) as it polarizes the Br-Br bond. The polarization causes polarization causes the bromine atoms within the Br-Br bond to become more electrophillic. The presence of Br+ compared to Br2 alone is a much better electrophille that can then react with benzene.
.jpg?revision=1&size=bestfit&width=639&height=214)
As the bromine has now become more electrophillic after activation of a catalyst, an electrophillic attack by the benzene occurs at the terminal bromine of Br-Br-AlBr3. This allows the other bromine atom to leave with the AlBr3 as a good leaving group, AlBr4-.
.jpg?revision=1&size=bestfit&width=350&height=175)
.jpg?revision=1&size=bestfit&width=371&height=218)
After the electrophilic attack of bromide to the benzene, the hydrogen on the same carbon as bromine substitutes the carbocation in which resulted from the attack. Hence it being an electrophilic aromatic SUBSTITUTION. Since the by-product aluminum tetrabromide is a strong nucleophile, it pulls of a proton from the Hydrogen on the same carbon as bromine.
.jpg?revision=1&size=bestfit&width=481&height=224)
In the end, AlBr3was not consumed by the reaction and is regenerated. It serves as our catalyst in the halogenation of benzenes.
Dissociation Energies of Halogens and its Effect on Halogenation of Benzenes
The electrophillic bromination of benzenes is an exothermic reaction. Considering the exothermic rates of aromatic halogenation decreasing down the periodic table in the Halogen family. Flourination is the most exothermic and Iodination would be the least. Being so exothermic, a reaction of flourine with benzene is explosive! For iodine, electrophillic iodination is generally endothermic, hence a reaction is often not possible. Similar to bromide, chlorination would require the aid of an activating presence such as Alumnium Chloride or Ferric Chloride. The mechanism of this reaction is the same as with Bromination of benzene.
Nitration and sulfonation of benzene are two examples of electrophilic aromatic substitution. The nitronium ion (NO2+) and sulfur trioxide (SO3) are the electrophiles and individually react with benzene to give nitrobenzene and benzenesulfonic acid respectively.
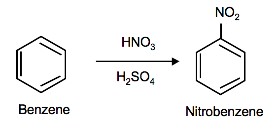
Nitration of Benzene
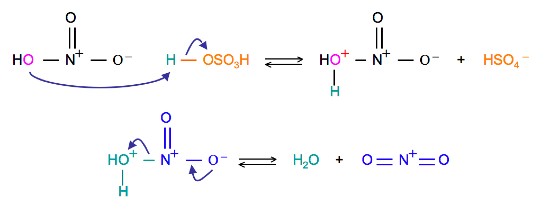
The source of the nitronium ion is through the protonation of nitric acid by sulfuric acid, which causes the loss of a water molecule and formation of a nitronium ion.
Sulfuric Acid Activation of Nitric Acid
The first step in the nitration of benzene is to activate HNO3with sulfuric acid to produce a stronger electrophile, the nitronium ion.
Because the nitronium ion is a good electrophile, it is attacked by benzene to produce Nitrobenzene.
Mechanism
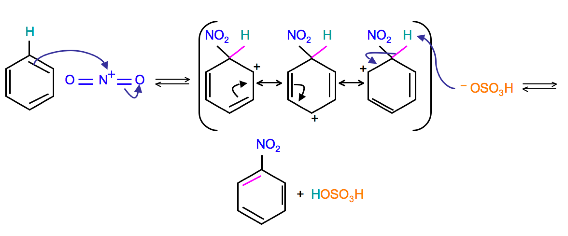
(Resonance forms of the intermediate can be seen in the generalized electrophilic aromatic substitution)
Sulfonation of Benzene
Sulfonation is a reversible reaction that produces benzenesulfonic acid by adding sulfur trioxide and fuming sulfuric acid. The reaction is reversed by adding hot aqueous acid to benzenesulfonic acid to produce benzene.
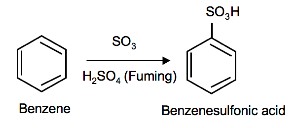
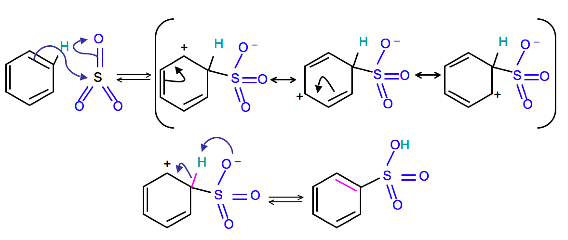
Mechanism
To produce benzenesulfonic acid from benzene, fuming sulfuric acid and sulfur trioxide are added. Fuming sulfuric acid, also refered to as oleum, is a concentrated solution of dissolved sulfur trioxide in sulfuric acid. The sulfur in sulfur trioxide is electrophilic because the oxygens pull electrons away from it because oxygen is very electronegative. The benzene attacks the sulfur (and subsequent proton transfers occur) to produce benzenesulfonic acid.
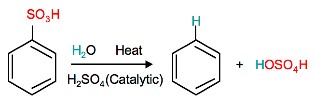
Reverse Sulfonation
Sulfonation of benzene is a reversible reaction. Sulfur trioxide readily reacts with water to produce sulfuric acid and heat. Therefore, by adding heat to benzenesulfonic acid in diluted aqueous sulfuric acid the reaction is reversed.
Further Applications of Nitration and Sulfonation
Nitration is used to add nitrogen to a benzene ring, which can be used further in substitution reactions. The nitro group acts as a ring deactivator. Having nitrogen present in a ring is very useful because it can be used as a directing group as well as a masked amino group. The products of aromatic nitrations are very important intermediates in industrial chemistry.
Because sulfonation is a reversible reaction, it can also be used in further substitution reactions in the form of a directing blocking group because it can be easily removed. The sulfonic group blocks the carbon from being attacked by other substituents and after the reaction is completed it can be removed by reverse sulfonation. Benzenesulfonic acids are also used in the synthesis of detergents, dyes, and sulfa drugs. Bezenesulfonyl Chloride is a precursor to sulfonamides, which are used in chemotherapy.
Exercises
Exercise \(\PageIndex{1}\)
1. What is/are the required reagent(s)for the following reaction:

- Answer
-
SO3 and H2SO4 (fuming)
Exercise \(\PageIndex{2}\)
What is the product of the following reaction:

- Answer
-

Exercise \(\PageIndex{3}\)
Why is it important that the nitration of benzene by nitric acid occurs in sulfuric acid?
- Answer
-
Sulfuric acid is needed in order for a good electrophile to form. Sulfuric acid protonates nitric acid to form the nitronium ion (water molecule is lost). The nitronium ion is a very good electrophile and is open to attack by benzene. Without sulfuric acid the reaction would not occur.
Exercise \(\PageIndex{4}\)
Write a detailed mechanism for the sulfonation of benzene, including all resonance forms.
- Answer
-

Exercise \(\PageIndex{5}\)
Draw an energy diagram for the nitration of benzene. Draw the intermediates, starting materials, and products. Label the transition states.
- Answer
-

Exercise \(\PageIndex{6}\)
In each case, how many products would be possible for the bromination of p-xylene, o-xylene, and m-xylene?
- Answer
-

Exercise \(\PageIndex{7}\)
If toluene is treated with D2SO4 all the hydrogen’s are replaced with deuterium. Explain.
- Answer
-
The deuterium is added to the ring. When the ring “re-aromatizes” the base scavenges the hydrogen before the deuterium and therefore is left on the ring. Continues for the rest of the hydrogen on the ring.

References
- Laali, Kenneth K., and Volkar J. Gettwert. “Electrophilic Nitration of Aromatics in Ionic Liquid Solvents.” The Journal of Organic Chemistry 66 (Dec. 2000): 35-40. American Chemical Society.
- Malhotra, Ripudaman, Subhash C. Narang, and George A. Olah. Nitration: Methods and Mechanisms. New York: VCH Publishers, Inc., 1989.
- Sauls, Thomas W., Walter H. Rueggeberg, and Samuel L. Norwood. “On the Mechanism of Sulfonation of the Aromatic Nucleus and Sulfone Formation.” The Journal of Organic Chemistry 66 (1955): 455-465. American Chemical Society.
- Vollhardt, Peter. Organic Chemistry : Structure and Function. 5th ed. Boston: W. H. Freeman & Company, 2007.


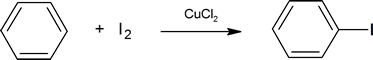
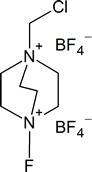
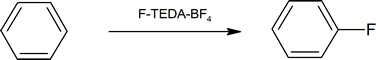
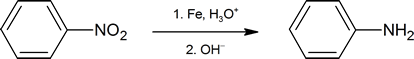
.jpg?revision=1&size=bestfit&width=272&height=170)

.jpg?revision=1&size=bestfit&width=639&height=214)
.jpg?revision=1&size=bestfit&width=350&height=175)
.jpg?revision=1&size=bestfit&width=371&height=218)
.jpg?revision=1&size=bestfit&width=481&height=224)





