2.2: Atomic Structure
- Page ID
- 221331
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- State the modern atomic theory.
- Learn how atoms are constructed.
The smallest piece of an element that maintains the identity of that element is called an atom. Individual atoms are extremely small. It would take about fifty million atoms in a row to make a line that is 1 cm long. The period at the end of a printed sentence has several million atoms in it. Atoms are so small that it is difficult to believe that all matter is made from atoms—but it is.
The concept that atoms play a fundamental role in chemistry is formalized by the modern atomic theory, first stated by John Dalton, an English scientist, in 1808. It consists of three parts:
- All matter is composed of atoms.
- Atoms of the same element are the same; atoms of different elements are different.
- Atoms combine in whole-number ratios to form compounds.
These concepts form the basis of chemistry. Although the word atom comes from a Greek word that means "indivisible," we understand now that atoms themselves are composed of smaller parts called subatomic particles. The first part to be discovered was the electron, a tiny subatomic particle with a negative charge. It is often represented as e−, with the right superscript showing the negative charge. Later, two larger particles were discovered. The proton is a more massive (but still tiny) subatomic particle with a positive charge, represented as p+. The neutron is a subatomic particle with about the same mass as a proton, but no charge. It is represented as either n or n0. We now know that all atoms of all elements are composed of electrons, protons, and (with one exception) neutrons. Table \(\PageIndex{1}\) summarizes the properties of these three subatomic particles.
| Name | Symbol | Mass (approx.; kg) | Charge |
|---|---|---|---|
| Proton | p+ | 1.6 × 10−27 | 1+ |
| Neutron | n, n0 | 1.6 × 10−27 | none |
| Electron | e− | 9.1 × 10−31 | 1− |
How are these particles arranged in atoms? They are not arranged at random. Experiments by Ernest Rutherford in England in the 1910s pointed to a nuclear model with atoms that has the protons and neutrons in a central nucleus with the electrons in orbit about the nucleus. The relatively massive protons and neutrons are collected in the center of an atom, in a region called the nucleus of the atom (plural nuclei). The electrons are outside the nucleus and spend their time orbiting in space about the nucleus. (Figure \(\PageIndex{1}\)).
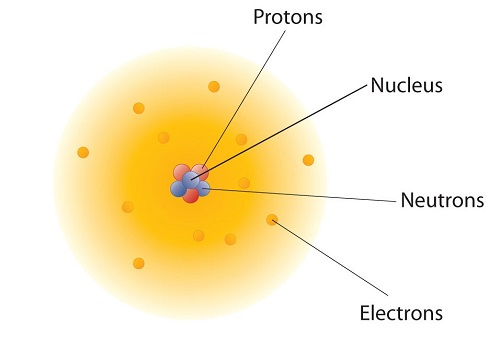
The modern atomic theory states that atoms of one element are the same, while atoms of different elements are different. What makes atoms of different elements different? The fundamental characteristic that all atoms of the same element share is the number of protons. All atoms of hydrogen have one and only one proton in the nucleus; all atoms of iron have 26 protons in the nucleus. This number of protons is so important to the identity of an atom that it is called the atomic number. The number of protons in an atom is the atomic number of the element. Thus, hydrogen has an atomic number of 1, while iron has an atomic number of 26. Each element has its own characteristic atomic number.
Atoms of the same element can have different numbers of neutrons, however. Atoms of the same element (i.e., atoms with the same number of protons) with different numbers of neutrons are called isotopes. Most naturally occurring elements exist as isotopes. For example, most hydrogen atoms have a single proton in their nucleus. However, a small number (about one in a million) of hydrogen atoms have a proton and a neutron in their nuclei. This particular isotope of hydrogen is called deuterium. A very rare form of hydrogen has one proton and two neutrons in the nucleus; this isotope of hydrogen is called tritium. The sum of the number of protons and neutrons in the nucleus is called the mass number of the isotope.
Neutral atoms have the same number of electrons as they have protons, so their overall charge is zero. However, as we shall see later, this will not always be the case.
Example \(\PageIndex{1}\):
- The most common carbon atoms have six protons and six neutrons in their nuclei. What are the atomic number and the mass number of these carbon atoms?
- An isotope of uranium has an atomic number of 92 and a mass number of 235. What are the number of protons and neutrons in the nucleus of this atom?
Solution
- If a carbon atom has six protons in its nucleus, its atomic number is 6. If it also has six neutrons in the nucleus, then the mass number is 6 + 6, or 12.
- If the atomic number of uranium is 92, then that is the number of protons in the nucleus. Because the mass number is 235, then the number of neutrons in the nucleus is 235 − 92, or 143.
Exercise \(\PageIndex{1}\)
The number of protons in the nucleus of a tin atom is 50, while the number of neutrons in the nucleus is 68. What are the atomic number and the mass number of this isotope?
- Answer
-
Atomic number = 50, mass number = 118
When referring to an atom, we simply use the element's name: the term sodium refers to the element as well as an atom of sodium. But it can be unwieldy to use the name of elements all the time. Instead, chemistry defines a symbol for each element. The atomic symbol is a one- or two-letter representation of the name of an element. By convention, the first letter of an element's symbol is always capitalized, while the second letter (if present) is lowercase. Thus, the symbol for hydrogen is H, the symbol for sodium is Na, and the symbol for nickel is Ni. Most symbols come from the English name of the element, although some symbols come from an element's Latin name. (The symbol for sodium, Na, comes from its Latin name, natrium.) Table \(\PageIndex{2}\) lists some common elements and their symbols. You should memorize the symbols in Table \(\PageIndex{2}\), as this is how we will be representing elements throughout chemistry.
| Element Name | Symbol | Element Name | Symbol |
|---|---|---|---|
| Aluminum | Al | Mercury | Hg |
| Argon | Ar | Molybdenum | Mo |
| Arsenic | As | Neon | Ne |
| Barium | Ba | Nickel | Ni |
| Beryllium | Be | Nitrogen | N |
| Bismuth | Bi | Oxygen | O |
| Boron | B | Palladium | Pd |
| Bromine | Br | Phosphorus | P |
| Calcium | Ca | Platinum | Pt |
| Carbon | C | Potassium | K |
| Chlorine | Cl | Radium | Ra |
| Chromium | Cr | Radon | Rn |
| Cobalt | Co | Rubidium | Rb |
| Copper | Cu | Scandium | Sc |
| Fluorine | F | Selenium | Se |
| Gallium | Ga | Silicon | Si |
| Germanium | Ge | Silver | Ag |
| Gold | Au | Sodium | Na |
| Helium | He | Strontium | Sr |
| Hydrogen | H | Sulfur | S |
| Iodine | I | Tantalum | Ta |
| Iridium | Ir | Tin | Sn |
| Iron | Fe | Titanium | Ti |
| Krypton | Kr | Tungsten | W |
| Lead | Pb | Uranium | U |
| Lithium | Li | Xenon | Xe |
| Magnesium | Mg | Zinc | Zn |
| Manganese | Mn | Zirconium | Zr |
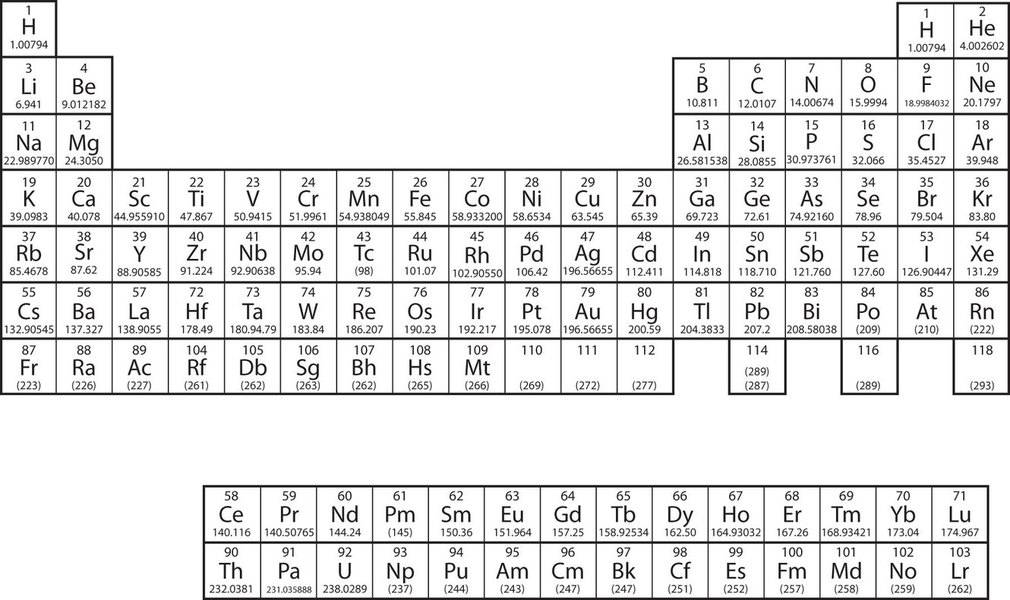
The elements are grouped together in a special chart called the periodic table of all the elements. A simple periodic table is shown in Figure \(\PageIndex{2}\), while one may view a more extensive periodic table from another source. The elements on the periodic table are listed in order of ascending atomic number. The periodic table has a special shape that will become important to us when we consider the organization of electrons in atoms (Chapter 8). One immediate use of the periodic table helps us identify metals and nonmetals. Nonmetals are in the upper right-hand corner of the periodic table, on one side of the heavy line splitting the right-side part of the chart. All other elements are metals.
There is an easy way to represent isotopes using the atomic symbols. We use the construction:
\[\ce{_{Z}^{A}X}\nonumber \]
where \(X\) is the symbol of the element, \(A\) is the mass number, and \(Z\) is the atomic number. Thus, for the isotope of carbon that has 6 protons and 6 neutrons, the symbol is:
\[\ce{_{6}^{12}C}\nonumber \]
where \(C\) is the symbol for the element, 6 represents the atomic number, and 12 represents the mass number.
Example \(\PageIndex{2}\):
- What is the symbol for an isotope of uranium that has an atomic number of 92 and a mass number of 235?
- How many protons and neutrons are in \(\ce{_{26}^{56}Fe}\)
Solution
- The symbol for this isotope is \(\ce{_{92}^{235}U}\)
- This iron atom has 26 protons and 56 − 26 = 30 neutrons.
Exercise \(\PageIndex{2}\)
How many protons are in \(\ce{_{11}^{23} Na}\)
- Answer
-
11 protons
It is also common to state the mass number after the name of an element to indicate a particular isotope. Carbon-12 represents an isotope of carbon with 6 protons and 6 neutrons, while uranium-238 is an isotope of uranium that has 146 neutrons.
A Short History of the Atomic Structure
The basic idea that matter is made up of tiny indivisible particles is very old, appearing in many ancient cultures such as Greece and India. The word atom is derived from the ancient Greek word atomos, which means "indivisible". This ancient idea was based on philosophical reasoning rather than scientific reasoning, and modern atomic theory was developed throughout a few centuries of research and experimentation.
The first atomic theory based on experimentation was stated by John Dalton, an English scientist, in 1808. By studying the chemical composition of different oxides, Dalton noticed that elements combined in very specific patterns given by small whole numbers. This observation prompted him to support that all matter is made of atoms, although Dalton had no idea about the actual structure of the atoms.
In 1897, J. J. Thomson discovered that the existence of the first subatomic particle. He renamed these new negatively charged particles as electrons. To explain the overall neutral charge of the atom, Thomson concluded that these electrons must be embedded in an uniform sea of positive charge. In this "plum pudding atomic model", the electrons were seen as embedded in the positive charge like raisins in a plum pudding.
Between 1908 and 1913, Ernest Rutherford and his colleagues Hans Geiger and Ernest Marsden came to have doubts about the Thomson model. Rutheford performed a series of experiments in which they bombarded thin foils of metal with positively charged alpha particles. They spotted alpha particles being deflected by angles greater than 90°. To explain this, Rutherford proposed that the positive charge of the atom is not distributed throughout the atom's volume as Thomson believed, but is concentrated in a tiny nucleus at the center. Rutherford's atomic model introduced the existence of the positively charged nucleus surrounded by the negatively charged electrons.
In 1913, after studying atomic spectra produced by elements. the physicist Niels Bohr proposed a model in which the electrons of an atom were assumed to orbit the nucleus but could only do so in a finite set of orbits, and could jump between these orbits only in discrete changes of energy corresponding to absorption or radiation of a photon. This quantized atomic model, also known as the planetary model of the atom, was used to explain why the electrons' orbits are stable and why elements absorb and emit electromagnetic radiation in discrete lines. The Bohr model of the atom was the first complete physical model of the atom. It described the overall structure of the atom and how atoms bond to each other. Bohr's planetary atomi model was not perfect and was soon superseded by the more accurate Schrödinger model, but it is sufficient to understand most chemical and physical properties discussed in this course.
In 1924, Louis de Broglie had proposed that all particles behave like waves to some extent, including the electron. In 1926 Erwin Schrödinger used this idea to develop the Schrödinger model of the atom. In this model, electrons are considered electromagnetic waves mechanics rather than particles. According to this model, it is mathematically impossible to obtain precise values for both the position and the energy (momentum) of an electron at a given point in time. This idea became the uncertainty principle, formulated by Werner Heisenberg in 1927. Because it is impossible to determine the position and the energy of an electron in an atom, Schrödinger's atomic model is based on the "probability" of finding an electron in a certain region around the nucleus. Thus, the orbits in the planetary model of the atom were discarded in favor of atomic orbitals, which are zones around the nucleus where a given electron is most likely to be observed.

Figure \(\PageIndex{2}\):A summary of atomic models. Image by Compound Interest (2016) https://www.compoundchem.com/2016/10/13/atomicmodels/. Shared under CC BY-NC-ND 4.0 license
Key Takeaways
- Chemistry is based on the modern atomic theory, which states that all matter is composed of atoms.
- Atoms themselves are composed of protons, neutrons, and electrons.
- Each element has its own atomic number, which is equal to the number of protons in its nucleus.
- Isotopes of an element contain different numbers of neutrons.
- Elements are represented by an atomic symbol.
- The periodic table is a chart that organizes all the elements.
Citations and attributions
" Atomic Theory" by LibreTexts is licensed under CC BY-NC-SA .
Wikipedia contributors. (2021, May 6). Atom. In Wikipedia, The Free Encyclopedia. Retrieved 15:20, May 18, 2021, from https://en.wikipedia.org/w/index.php?title=Atom&oldid=1021695004
Wikipedia contributors. (2021, May 8). J. J. Thomson. In Wikipedia, The Free Encyclopedia. Retrieved 15:21, May 18, 2021, from https://en.wikipedia.org/w/index.php?title=J._J._Thomson&oldid=1022092604

