Accelerator Mass Spectroscopy
- Page ID
- 202053
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Accelerator Mass Spectroscopy (AMS) is a highly sensitive technique that is useful in isotopic analysis of specific elements in small samples (1mg or less of sample containing 106 atoms or less of the isotope of interest).[1]
Accelerator Mass Spectroscopy
AMS requires a particle accelerator, originally used in nuclear physics research, which limits its widespread use due to high costs and technical complexity. Fortunately, UC Davis researchers have access to the Lawrence Livermore National Laboratory Center for Accelerator Mass Spectrometry (CAMS LLNL), one of over 180 AMS research facilities in the world. AMS is distinct from conventional Mass Spectrometry (MS) because it accelerates ions to extremely high energies (millions of electron volts) compared to the thousands of electron volts in MS (1keV=1.6×10-16 J). This allows AMS to resolve ambiguities that arise in MS due to atomic and molecular ions of the same mass. AMS is most widely used for isotope studies of 14C, which has applications in a variety of fields such as radiocarbon dating, climate studies, and biomedical analysis.[2] Some of the most fascinating applications of AMS range from exposure dating of surface rocks, 14C labeled drug tracer studies, and even radiocarbon dating of artifacts such as the Shroud of Turin and the Dead Sea Scrolls.[3]
Theory
In conventional atomic mass spectrometry, samples are atomized and ionized, separated by their mass-to-charge ratio, then measured and/or counted by a detector. Rare isotopes such as 14C present a challenge to conventional MS due to their low natural abundance and high background levels. Researchers were challenged by isobaric interference (interference from equal mass isotopes of different elements exemplified by 14N in 14C analysis), isotopic interference (interference from equal mass to charge isotopes of different elements), and molecular interference (interference from equal mass to charge molecules, such as 12CH2-, 12CD, or 13CH- in 14C analysis). Most AMS systems employ an electrostatic tandem accelerator that has a direct improvement in background rejection, resulting in a 108 time increase in the sensitivity of isotope ratio measurements. As the natural abundance of 14C in modern carbon is 10-12 (isotopic ratio of 14C:12C), a sensitivity of 10-15 is a prerequisite for 14C analysis.
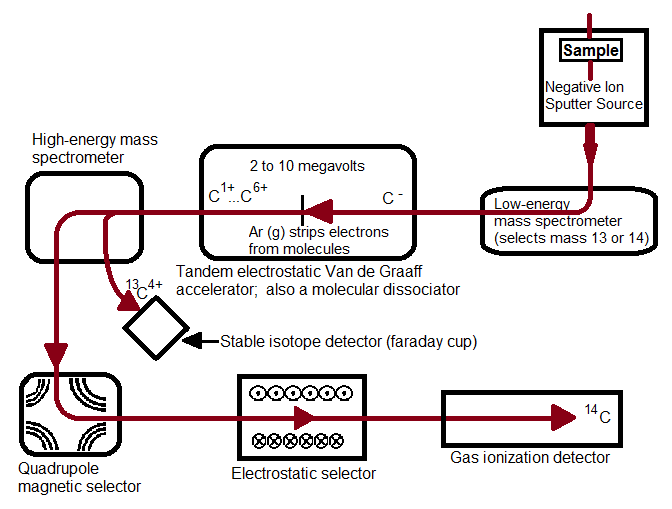
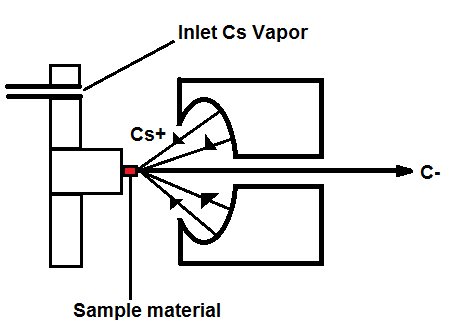
Figure 1, above, starts with a negative ion sputter source, which commonly consists of a stream of Cesium ions (Cs+) with energies of 2-3 keV focused on the surface of a solid sample in order to transfer enough energy to the target material to produce free atoms and ions of the sample material. This process, called sputtering, separates neutral, as well as positive and negative ions from the sample surface. The sample is held at a negative potential, and negatively charged ions are accelerated away from the sample, resulting in a beam of negative ions (Figure 2, below). Cs+ is particularly useful in 14C studies because it does not form a negative ion from 14N, thereby eliminating isobar interference.[4] It is important to have a beam of negative ions entering the accelerator because the negative ions are attacted to the high -voltage terminal which results in their net acceleration.
The low energy (~5-10 keV) diverging beam that leaves the ion source is accelerated, focused and transported to the accelerator by the injector system.[2] CAMS LLNL employs a low-energy mass spectrometer that selects for the desired atomic mass[5] that separates ions by their mass to charge ratio (12C, 13C, and 14C ions pass through separately). Most AMS systems use sequential injection, a process that switches between stable and rare isotopes via the application of varying voltages to the electrically insulated vacuum chamber of the analyzer magnet. In sequential injection, typical injection repetition rates are 10 sec-1 to minimize variations in the electrical load.[2] This process allows the development of more versatile systems, allowing for analysis of a wide range of isotopes.[1] The alternative to sequential injection is simultaneous injection, a process adopted in accelerators dedicated to 14C analysis. A recombinator is used following sequential injection, which is a sequence of magnetic analyzers and quadrupole lenses that focus the stable and rare isotopes so they recombine and enter the accelerator together.
The traditional accelerator was first developed in the early 1930s for nuclear physics research. In 1939, UC Berkeley scientists Luis Alvarez and Robert Cornog were the first to use AMS in the detection of 3He in nature using the 88-inch Berkeley cylclotron.[5] Now, over 70 years later, cyclotrons have been replaced by an accelerator type with greater energy stability: the tandem electrostatic accelerator. An electrostatic accelerator works by accellerating particles though a magnetic field generated by high voltages using a mechanic transport system that continuously transports charges from ground to the insulated high-voltage terminal. All tandem accelerators with a maximum terminal voltage above 5 MV use such a mechanical system.[2] The negative ions that enter the accelerator are attracted to the high-voltage terminal, which is what accellerates theCAMS LLNL employs a tandem Van de Graaff accelerator, in which a second acceleration of millions of volts is applied. In all tandem accelerators, atoms are stripped at the high-voltage terminal using either a thin Carbon foil or Argon gas. Stripping is the process in which two or more electrons are removed. The Van de Graaff accelerator removes at least four electrons. It is preferrable to remove at least three electrons because by this process that molecular isobars of 14C (such as 12CH2-, 12CD, or 13CH-) are destroyed due to the high instability of their positively charged forms, and atomic C+ ions such as 12C+, 13C+, and 14C+ are separated due to their different mass to charge ratios.[4] The negative ions are changed to positively charged ions and are thus accelerated back to the ground potential in the high-energy part of the accelerator. Transmission through a foil changes with time due to radiation damage and foil thickening, thus gas strippers are used in all modern analyzers due to their increased transmission stability.[2]
Magnetic lenses focus the high energy particles leaving the accelerator into a magnetic dipole, (the high energy analyzing magnet). Stable isotopes can be collected at off-axis beam stops where secondary focusing lenses and additional analyzing equipment remove unwanted ions and molecular fragments to eliminate background. At CAMS LLNL, a magnetic quadrupole lens focuses the desired isotope and charge state to a high-energy mass spectrometer which passes 12C+ and 13C+ into Faraday cups and further focuses and stabilizes 14C in a quadrupole/electrostatic cylindrical analyzer that leads to a gas ionization detector.[5] The magnetic quadrupole and electrostatic selectors coupled together ensure high selectivity and sensitivity, respectively. Other detectors commonly found in AMS systems include surface barrier, time-of-flight, gas filled magnets, and x-ray detectors.
Interpretation
Rare isotopes analyzed by AMS are always measured as a ratio of a stable, more abundant (but not too abundant) isotope. For example, the ratio in 14C studies is generally shown as 14C/13C. Less abundant isotopes are preferable in AMS because the decreased flux of ions reduces background and wear on the instrument, which is of particular concern due to the quick deterioration of particle detectors (performance deteriorates at rates higher than a few thousand particles per second[1]).
Applications
Common radioisotope elements measured with AMS and their applications are shown in Table 1[4], below. Because 14C analysis is by far the most popular application of AMS, the methods discussed below are all techniques used involving 14C.
| Element (Common Isotope) | Radioisotope with AMS | Natural abundance | Half-life (yr) | Study application |
|---|---|---|---|---|
| Hydrogen (1H) | 3H | trace | 12.33 | Biological/biomedical Nutritional trace |
| Beryllium (9Be) | 10Be | trace | 1,510,000 | Geochronology Hydrogeological study Exposure dating |
| Carbon (12C) | 14C | 1 x10-10% | 5730 | Biological/biomedical Nutritional trace |
| Aluminum (27Al) | 26Al | trace, synthetic | 720,000 | Biological/biomedical Exposure dating |
| Chlorine (35Cl) | 36Cl | 7x10−11% | 301,000 | Earth Science Hydrogeological study Exposure dating Migration of nuclear waste |
| Calcium (40Ca) | 41Ca | trace, synthetic | 116,000 | Biological/biomedical Nuclear weapon testing |
| Nickel (58Ni) | 59Ni | trace, synthetic | 112,000 | Nutritional trace |
| Iodine (127I) | 129I | trace, synthetic | 15,700,000 | Biological/biomedical Migration of nuclear waste Environmental study |
Radiocarbon dating is an analytical method based on the rate of decay of 14C, a radioactive carbon isotope formed in the atmosphere by the reaction between neutrons from cosmic rays and 14N (neutron + 14N = 14C + proton).[2] Resultant 14C atoms are taken up by plants in the form of 14CO2, then transferred to animals though the food chain. When animals and plants die, they cease to uptake 14C, and a steady decay of 14C continues in their tissues over time. 14C atoms decay via electron emission (β radiation) to form 14N, a process which has a half life of 5,730 years.[5] Radiocarbon levels in the atmosphere change according to complex patterns which are affected by a variety of fluctuations ranging from the sun’s solar activity and the earth’s magnetic field, to ocean ventilation rate and climate. 14C analysis of tree rings, corals, lake sediments, ice cores, and other sources has led to a detailed record of 14C variations through time, allowing researchers to establish an official radiocarbon calibration curve (also referred to as a radiocarbon clock) dating back 26,000 calendar years. In the 1960s, nuclear weapons testing released large amounts of neutrons into the atmosphere, nearly doubling 14C activity.[2] Samples taken after this time period can be radiocarbon dated using a 14C bomb curve like the peak shown below in Figure 3, can retrieve very precise dates (within 1 year at the steepest part of the curve).
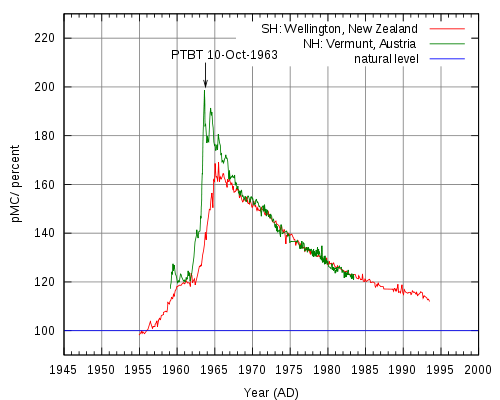
14C analysis provides valuable information in the radiocarbon dating of the world’s most priceless artifacts. One such example of the monumental impact of 14C AMS is the radiocarbon dating of the Dead Sea Scrolls to dates from 300 BC to AD 61 by labs in Zurich and Arizona. AMS has also contributed greatly to environmental and atmospheric studies by providing information regarding particle composition and origin. In the biochemical field, synthesized 14C labeled compounds can be administered as a tracer dose for in-vivo human metabolic and drug studies which require AMS analysis of graphitized biological samples.
AMS is a highly sensitive method for isotopic analysis that has numerous key applications that are only growing with advances in technology. High costs and technical complexities that arise with the use of a particle accelerator are the only limits to the widespread use of AMS. Recent times have seen the emergence of commercially available compact accelerators that use as low as 200 kV for radiocarbon dating and biomedical applications, and as particle accelerators become more commonplace, modifications to the instrument have also broadened the number of isotopes the instrument can measure.
References
- Tuniz, C., Bird, J. R., Fink, D. and G. F. Herzog, Accelerator Mass Spectrometry. CRC PRess: Boca Raton, 1998; p 371.
- Hellborg, R.; Skog, G., Accelerator mass spectrometry. Mass Spectrometry Reviews 2008, 27 (5), 398-427.
- Gove, H. E., From Heroshima to the Iceman. Institute of Physics Publishing: London, 1999; p 225.
- Kim, S.. Graphitization for biological, biomedical, and environmental carbon-14-accelerator mass spectrometry applications: Optimization and characterization. Ph.D. dissertation, University of California, Davis, United States -- California. Retrieved February 28, 2011, from Dissertations & Theses @ University of California.(Publication No. AAT 3379582).
- https://www.llnl.gov/str/Holloway.htmlc
Contributors
- Laura McWade

