3.1: Building Blocks
- Page ID
- 414160
It would be difficult to identify a scientific principle that is as widely known and universally accepted as the notion that all matter is composed of atoms. It may come as a surprise, then, that up until the early 1900s the idea that matter was so constituted was not firmly established – many talented scientists were skeptical of such a view. [1] Why was this? Ask yourself: “Why should I believe in the existence of atoms? What evidence is there for them?” Most people unquestioningly accept the notion that atoms are real yet would have difficulty answering these questions. Over the years we have posed just this sort of question to students, many of whom express exasperation at the effrontery of it. “It’s common sense!” is a frequent response. What is clear from this and similar answers is that the notion that matter is, in fact, atomic in nature is so deeply ingrained that an alternative view is hard to imagine. The historically preferred view has been that matter is continuous, meaning that it could be divided indefinitely, always yielding smaller bits of matter that could be further divided without losing their essential nature. This clearly differs from a particulate view of matter, but our senses give no clue as to which view, if either, is correct. Atoms exist, for us, only in the abstract. It is, therefore, decidedly not common sense that leads most people to subscribe to the atomic view of matter as no direct sensory experience supports it.
The establishment of atomic theory represents a triumph of the scientific method and is an enthralling story, one that we encourage you to investigate critically. Greek philosophers, particularly Democritus, first proposed an atomic view of matter, but those ideas were not experimentally tested and, perhaps more importantly, were opposed by Aristotle whose followers essentially buried the concept for about two thousand years. It wasn’t until scientists such as Boyle, Lavoisier, Proust, Cavendish, and Priestley, working in the intellectual milieu that defined The Age of Enlightenment, began systematically studying and experimenting with matter did clues to its corpuscular nature emerge. None of these scientists subscribed to any sort of atomic view of matter, however, as Aristotle’s resistance to the concept was deeply rooted indeed. It wasn’t until the English scientist and educator John Dalton (Figure 3-1) synthesized several distinct  principles propounded by some of the aforementioned scientists and proposed the first modern atomic theory based on physical evidence. Specifically, Antoine Lavoisier, through many careful and painstaking measurements using instruments of his own design, discovered that matter is neither created nor destroyed during chemical reactions [2]; we refer to this as the Law of Conservation of Mass. Joseph Proust, on the other hand, discovered the Law of Definite Proportions, meaning that pure compounds always exhibit the same mass ratios of their component elements; water, for example, is always 88.8% oxygen and 11.2% hydrogen by weight.
principles propounded by some of the aforementioned scientists and proposed the first modern atomic theory based on physical evidence. Specifically, Antoine Lavoisier, through many careful and painstaking measurements using instruments of his own design, discovered that matter is neither created nor destroyed during chemical reactions [2]; we refer to this as the Law of Conservation of Mass. Joseph Proust, on the other hand, discovered the Law of Definite Proportions, meaning that pure compounds always exhibit the same mass ratios of their component elements; water, for example, is always 88.8% oxygen and 11.2% hydrogen by weight.
Figure 3-1. An engraving of John Dalton (by William Henry Worthington); the papers on the desk are meant to resemble Dalton's original illustrations he used when explaining his atomic theory in his 1808 book A New System of Chemical Philosophy.
Problem 3.1 Using their atomic masses, show that pure water, H2O, is 88.8% oxygen and 11.2% hydrogen by weight.
Solution
This is a matter of finding the mass of each element in a compound relative to the total mass. The mass percent of each is calculated as shown below:
\[ weight\ percent\ oxygen = \frac{mass\ oxygen\ atoms}{molecular\ mass} * 100\%
= \frac{15.999 amu}{15.999 amu + 2 * 1.0079 amu} * 100\% = 88.81\% \nonumber\]
\[ weight\ percent\ hydrogen = \frac{mass\ hydrogen\ atoms}{molecular\ mass} * 100\%
= \frac{2 * 1.0079 amu}{15.999 amu + 2 * 1.0079 amu} * 100\% = 11.19\% \nonumber \]
Problem 3.2 Using their atomic masses, calculate the weight percent of carbon, hydrogen and oxygen in linoleic acid, C18H32O2.
Problem 3.3 Demonstrate your understanding of the Law of Definite Proportions by explaining how it applies to linoleic acid using your result from Problem 3-1.
Dalton reasoned that Lavoisier’s and Proust’s important observations were both consistent with a particulate view of matter. His elegant explanation posited that compounds consisted of specific whole number ratios of fundamental particles of each element, and that atoms of each element were identical to each other, but had masses that were different from those of other elements. The Law of Definite Proportions falls out naturally from those assumptions. Furthermore, he suggested that chemical reactions were, in essence, simple rearrangements of the component atoms of the starting compounds – they changed partners, but their existence per se was not affected by the transformations taking place, hence their total mass is not changed upon reaction (Figure 3-2). Dalton’s model was overly simple, particularly with respect to atomic structure as we discuss in the next section, but his atomic theory is rightfully viewed as a monumental step forward in our understanding of matter.
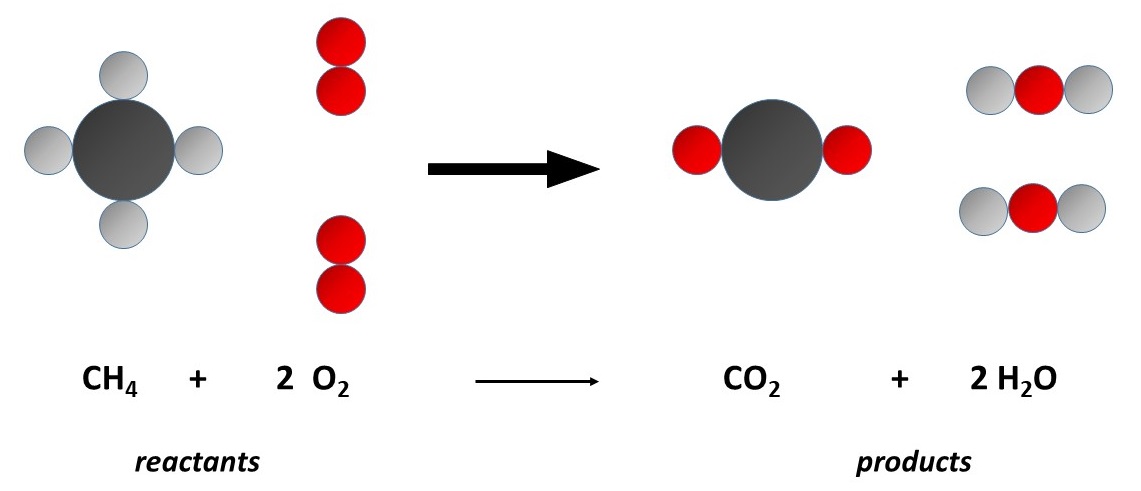
Figure 3-2. The combustion of methane as viewed by Dalton's atomic theory:
1) The Conservation of Mass is consistent with atoms retaining their original masses and quantities during chemical reactions.
2) Law of Definite Proportions is a consequence of methane, for example, as always consisting of a 4:1 ratio of its component atoms.

Figure 3-3. Two bars of pure gold, each with a mass of 1.000 kilograms. (Photo by Ariel Palmon CC BY-SA 3.0)
If we accept Dalton’s idea that matter is atomic in nature, other questions naturally emerge: What is an atom like? What is its nature? Let's begin with a simple example: a 1000 g gold bar that you could hold in your hands and describe in detail (Figure 3-3). You would immediately notice that it is relatively heavy for its size, that is, it is quite dense [3], and has the beautiful color and luster that has made it a prized material since antiquity. Pure gold is an example of an element, a material that consists of one and only one type of atom, in accord with Dalton’s theory, making it the purest form of matter. Now, imagine dividing this 1000 g bar of gold in half: you would have two pieces of gold with identical physical characteristics, but each would have only half the mass of the original, 500 grams each. According to the continuous view of matter, one could repeat this process infinitely where, with each division the mass would decrease by a factor of two but you would always have be left with samples of gold. In reality there is a limit to how many times you can divide the bar. At the beginning of the process the physical properties, e.g., the color, density, etc., of each partitioned sample would remain unchanged. After about 40 such bisections, however, the resulting particles of gold become barely visible to the naked eye, the size of a mere dust speck about 5 microns across [4]. But many more divisions are still possible. You will reach a point, after about 80 divisions of the original 1 kg bar, when you arrive at a single, indivisible piece of gold — a single atom — the “building block” of the element. Can this atom be divided further? Yes, but the result is no longer identifiable as gold. It would lose the properties and composition that define a gold atom as a gold atom. A rather macabre example may help here: I can have a litter of eight roly-poly puppies; taking half away, leaves me four; a second division leaves me two. Only one more division is possible, and it leaves me with a single, lovable puppy. If I continue to divide, what I get is no longer a puppy, much less a loveable one. Half a puppy is not a puppy. Likewise, half of a gold atom is not a gold atom (Figure 3-4). [5]
Figure 3-4. Puppies, like atoms, can't be divided and still retain their original properties. (Photo by by Lisa L Wiedmeier is licensed under CC BY-SA 2.0)
The above illustrates the relationship between atoms and elements that is sometimes overlooked by those beginning their study of chemistry. All elements, by definition, are samples of matter that are similarly composed of only one type of atom. Likewise, compounds, such as linoleic acid that we discussed in Chapter 1, consist of one and only one type of molecule. Each and every particle of linoleic acid, the individual molecules, are identical in that they consist of eighteen carbon, two oxygen, and thirty-two hydrogen atoms that are arranged in precisely the same way. They are also ridiculously small. How small? In one drop, roughly 0.1 mL, there would be about 200,000,000,000,000,000,000 of them. Thus you can see and feel the compound called linoleic acid, but it is beyond our ability to detect with any of our senses an individual molecule of that compound. Analogously, a single atom of gold is too small to notice, but we can observe the element called gold, and that would consist of unfathomably large numbers of individual gold atoms. That dust speck sized piece of gold we mentioned above would have about four trillion of them.
It is the truly incomprehensibly small scale of individual atoms and molecules that gives rise to their abstract nature. If we can’t see them, how can we know their shape, size, or other characteristics? Why discuss them at all? While such skepticism is (almost) always healthy, we ask that you set aside such contrarian impulses and bear with us as we describe atoms and how they behave in greater depth. We feel justified in making this modest request because chemists and physicists have generated a wealth of evidence that supports the atomic view of matter since Dalton first proposed it. The increasingly sophisticated models scientists use to predict the behavior of matter have proven to be amazingly accurate. If you want to understand how drugs work, how pollution can be addressed, how to design a device that can safely and efficiently store the energy from sunlight, or find solutions to any one of hundreds of other pressing problems, an understanding of atomic structure is essential.
Problem 3.4 Gold has a molar mass of 196.97 g/mol. Calculate how many gold atoms are in one of the bars shown in Figure 3-3. With a density of 19.3 g/cm3, calculate the volume of one of those bars in cm3. What would be the mass of a sample of water having the same volume?
Footnotes and References.
[1]. An interesting book on the development of atomic theory, focusing primarily on its development in the realm of physics, is Boltzmann’s Atom by David Lindley (2001).
[2] Antoine Lavoisier, who we already mentioned in Chapter 2, is generally considered the founder of modern chemistry; his discovery of The Conservation of Mass put chemistry on firm quantitative footing. Prior to his work it was widely held that matter could be destroyed, in combustion for instance. His careful measurements of the weights of gaseous reactants and products, not a trivial problem, showed that this is incorrect. Lavoisier was executed on the guillotine during the French Revolution, despite the fact that he did much to improve the living conditions in Paris before prior to that time.
[3] Recall from Chapter 2: density is defined as the mass to volume ratio of an object or material, or D = m/V. At 19.3 g/cm3, gold is one of the most dense elements; for comparison, water has a density of 1.00 g/mL, and that for aluminum, iron and lead are 2.70, 7.84, and 11.3 g/cm3, respectively.
[4] One micron (abbreviated μm) is equal to one one-thousandth of a millimeter, or one one-millionth of a meter (1×10-6 m); 5 μm is roughly the same diameter as a single strand of spider silk, which is finer than the finest human hair.
[5] The word atom comes from the Greek atomos, meaning indivisible. This mental exercise, that of imagining what might happen if a sample of matter was repeatedly halved, was first described by Democritus (460-370 BC), who is credited with being the first natural philosopher to articulate an atomic theory of matter. With respect to what is meant by the building block idea, an analogy that is similar to our puppy example may be helpful. Think about a flock of birds: a single bird is the “building block” of the entire flock. So, flock is to bird as element is to atom. If one starts with the flock, it could conceivably be serially divided until all that remains is a pair of birds – one last division then leaves a single bird. More divisions are indeed possible, but what you are left with is no longer the simplest unit of the entire flock - the building block. It is something: limbs, feathers and innards perhaps, but it is no longer a bird that, when taken many times, gives a flock. Rather, our subdivided bird taken many times gives a large but flightless collection of limbs, feathers and innards. Likewise, when you get to a single atom, further division is possible, but the result is no longer a building block of the element you started with.


