2.4: Vapor Pressure
- Page ID
- 164738
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Another important property of liquids (and solids) that is governed by intermolecular forces is vapor pressure. Vapor pressure is defined as the partial pressure of a substance in the gas phase (vapor) that exists above a sample of the liquid in a closed container. The phenomenon of vapor pressure is explained by the kinetic molecular theory again, which shows that a liquid always exists in equilibrium with its vapor.
Evaporation: Liquid/Vapor Equilibrium
The average energy of the particles in a liquid is governed by the temperature. The higher the temperature, the higher the average energy. But within that average, some particles have energies higher than the average, and others have energies lower than the average. Some of the more energetic particles on the surface of the liquid can be moving fast enough to escape from the attractive forces holding the liquid together. They evaporate. The diagram shows a small region of a liquid near its surface.
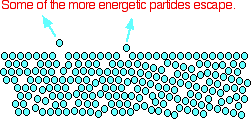
Notice that evaporation only takes place on the surface of the liquid. That's quite different from boiling which happens when there is enough energy to disrupt the attractive forces throughout the liquid. That's why, if you look at boiling water, you see bubbles of gas being formed all the way through the liquid.
If you look at water which is just evaporating in the sun, you don't see any bubbles. Water molecules are simply breaking away from the surface layer. Eventually, the water will all evaporate in this way. The energy which is lost as the particles evaporate is replaced from the surroundings. As the molecules in the water jostle with each other, new molecules will gain enough energy to escape from the surface.
Now imagine what happens if the liquid is in a closed container. Common sense tells you that water in a sealed bottle does not seem to evaporate - or at least, it does not disappear over time. But there is constant evaporation from the surface. Particles continue to break away from the surface of the liquid - but this time they are trapped in the space above the liquid.
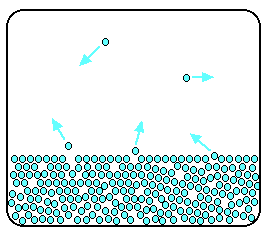
As the gaseous particles bounce around, some of them will hit the surface of the liquid again, and be trapped there. There will rapidly be an equilibrium set up in which the number of particles leaving the surface is exactly balanced by the number rejoining it.
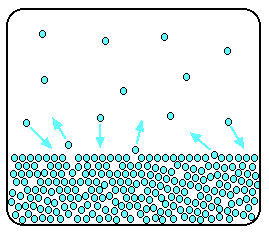
In this equilibrium, there will be a fixed number of the gaseous particles in the space above the liquid. When these particles hit the walls of the container, they exert a certain amount of pressure. This pressure is the vapor pressure (also known as saturated vapor pressure) of the liquid.
Vapor Pressure and Intermolecular Forces
Different substances have different vapor pressures at a given temperature due to the strength of their intermolecular forces. For molecules to escape into the vapor phase, they must have enough kinetic energy to overcome the intermolecular forces of other molecules at the surface of the liquid. It follows, therefore, that substances with weaker intermolecular forces will allow more molecules to escape into the gas phase at equilibrium - in other words, they will have a higher vapor pressure. Substances with strong intermolecular forces will have lower vapor pressure, because fewer molecules will have enough kinetic energy to escape at a given temperature.
Substances with high vapor pressures are said to be volatile - that is, they easily evaporate. Many small organic compounds are volatile, including most scent compounds. If you can smell a liquid, it is because molecules of the liquid are evaporating into the gas phase and traveling through the air to your nose!
Substances with strong intermolecular forces have lower vapor pressures and are less volatile, while substances with weak intermolecular forces have higher vapor pressures and are more volatile.
Example \(\PageIndex{1}\)
Formaldehyde (COH_2) and methanol (CH_3OH) are two small organic compounds of similar size. Which would be expected to have a higher vapor pressure at room temperature?
Solution
Consider the intermolecular forces at play in each molecule. The two compounds are of similar size and molecular weight, so they will exhibit similar dispersion forces. Formaldehyde is a polar molecule due to the C=O bond, so it will exhibit dipole-dipole forces. Methanol is also polar and also includes an O-H bond, which means it will exhibit hydrogen bonding. Because hydrogen bonding is the strongest type of IMF, we would expect methanol to have stronger IMF than formaldehyde.
Formaldehyde, with weaker IMF, should have a higher vapor pressure. (In fact, with a boiling point of -19 °C, formaldehyde exists as a gas at room temperature, while methanol exists as a liquid.)
Exercise \(\PageIndex{1}\)
Citronellal and citronellol are two related compounds found in citronella oils and used in perfumes and insect repellants. The molecules differ by one C=O bond in citronellal that is replaced by a C-O-H group in citronellol. Which compound would be expected to be more volatile?
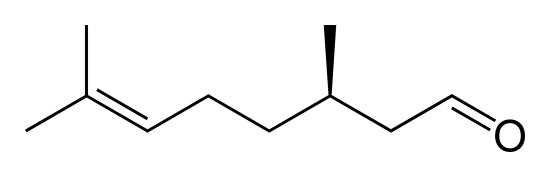
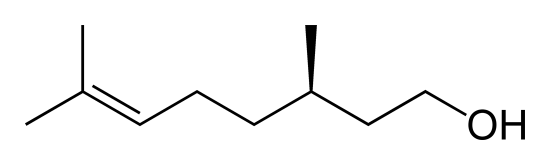
Citronellal (left) and citronellol (right)
- Answer
-
Citronellal would be more volatile due to the absence of hydrogen bonding.
Vapor Pressure and Temperature
According to the kinetic molecular theory, the temperature reflects the average kinetic energy of the particles in a liquid. If you increase the temperature, you are increasing the average energy of the particles present. That means that more of them are likely to have enough energy to escape from the surface of the liquid. That will tend to increase the vapor pressure.
The increase in vapor pressure with temperature is not linear, however. For example, consider the vapor pressure of water between 0°C and 100 °C, shown in Figure \(\PageIndex{1}\) below. As the temperature rises, the vapor pressure of water rises exponentially. In the next chapter, we will see how vapor pressure values can be predicted based on this relationship with temperature.
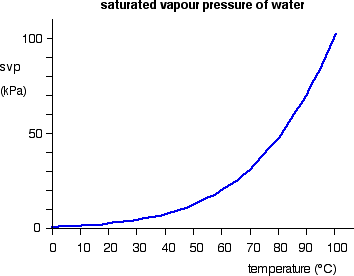
Figure \(\PageIndex{1}\). The graph shows how the saturated vapor pressure (svp) of water varies from 0°C to 100 °C. The pressure scale (the vertical one) is measured in kilopascals (kPa). 1 atmosphere pressure is 101.325 kPa.
The vapor pressure of a liquid increases nonlinearly with temperature.
Vapor pressure and boiling point
Notice in Figure \(\PageIndex{1}\) that at the vapor pressure of water at 100 °C is equal to 1 atm - normal atmospheric pressure. In fact, this is always true at the normal boiling point of a liquid. A liquid boils when its equilibrium vapor pressure becomes equal to the external pressure on the liquid. When that happens, it enables bubbles of vapor to form throughout the liquid - those are the bubbles you see when a liquid boils.
If the external pressure is higher than the saturated vapor pressure, these bubbles are prevented from forming, and you just get evaporation at the surface of the liquid. If the liquid is in an open container and exposed to normal atmospheric pressure, the liquid boils when its saturated vapor pressure becomes equal to 1 atmosphere (or 101325 Pa or 101.325 kPa or 760 mmHg). This happens with water when the temperature reaches 100°C.
At different external pressures, however, water will boil at different temperatures. For example, at the top of Mount Everest the pressure is so low that water will boil at about 70°C. Whenever we just talk about "the boiling point" of a liquid, we always assume that it is being measured at exactly 1 atmosphere pressure. In practice, of course, that is rarely exactly true.
Sublimation: solid/vapor Equilibrium
Solids can also lose particles from their surface to form a vapor, except that in this case we call the effect sublimation rather than evaporation. Sublimation is the direct change from solid to vapor (or vice versa) without going through the liquid stage.
In most cases, at ordinary temperatures, the saturated vapor pressures of solids range from low to very, very, very low. The forces of attraction in many solids are too high to allow much loss of particles from the surface. However, there are some which do easily form vapors. For example, naphthalene (used in old-fashioned "moth balls" to deter clothes moths) has quite a strong smell. Molecules must be breaking away from the surface as a vapor, because otherwise you would not be able to smell it. Another fairly common example (discussed in detail on another page) is solid carbon dioxide - "dry ice". This never forms a liquid at atmospheric pressure and always converts directly from solid to vapor. That's why it is known as dry ice.


