9.1: The Bonding Spectrum
- Page ID
- 98640
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Skills to Develop
- To identify ionic, covalent, and metallic bonding and understand the limits of each model.
The properties of all materials depend not only on the types of atoms that compose them but also on how those atoms are chemically bonded together. The nature of chemical bonding is perhaps the most central question of chemistry as a discipline: what is a chemical bond, and why do different atoms bond together in one way or another? There is no one “right” answer to this question—rather, chemists have developed different models that help us to understand chemical bonding in the substances we encounter. Researchers continue to debate the validity of these theories in different chemical situations as new discoveries are made. However, we can guide our discussion by stating a few general principles about chemical bonding:
-
Chemical bonds are formed due to the movement of electrons between or among the atomic nuclei. It is ultimately the electrostatic attraction between positive nuclei and negative electrons that holds chemicals together.
-
Chemical bonding always lowers the potential energy of the bonded system—we say that a bonded system is “more stable”. Forming a bond releases energy to the surroundings, while breaking a bond requires energy input.
In this section, we will examine three important models describing three classes of chemical bonding: ionic, covalent, and metallic. It is worth remembering that all of these models are simplifications of reality and not necessarily mutually exclusive. However, they can help us understand a great deal about how different elements combine and create the properties of materials that we observe.
Bonding Models
A successful model of chemical bonding must use what we know about the nature of atoms to account for the various properties of chemical substances that we observe. Based on quantum theory, we know that electrons occupy atomic orbitals with particular shapes and energies around the nucleus. Electrons may be removed from or added to an atom to form an ion, which involves an energy change (ionization energy or electron affinity). Because too much energy is required to remove electrons in core orbitals, only the electrons farthest from the nucleus—the valence electrons—will be able to be moved around between atoms to form chemical bonds.
Only valence electrons are involved in chemical bonding.
Moreover, we know that atoms exhibit periodic trends in their ability to gain or lose electrons that is based on their electron configuration and thus their placement in the periodic table. Most important for understanding bonding is the concept of electronegativity—how strongly an atom will attract electrons toward itself in a chemical bond. Recall that atoms become more electronegative toward the upper-right corner of the periodic table. Not coincidentally, this trend also parallels the classification of elements as metals, metalloids, and nonmetals, classifications that are ultimately based on the type of bonding each element exhibits.
Three major types of compounds can be described by three complementary bonding models, summarized below:
- Ionic Bonding: In ionic bonding, electrons are considered to be transferred completely from one atom to another atom (or group of atoms), forming ions of opposite charge. These ions then attract each other electrostatically to form a stable crystalline lattice. Because electrons must be transferred from one element to another, this type of bonding is commonly seen between elements with a large difference in electronegativity, such that one atom attracts the electron(s) completely away from the other. This is the case in compounds involving both a metal (low electronegativity) and a nonmetal (high electronegativity).
- Covalent Bonding: In covalent bonding, electrons are considered to be shared between atoms via the interaction of atomic orbitals. These interactions hold the atoms together by bonds with a particular direction and strength, forming network solids or molecules that then act as individual units of the compound. Covalent bonding is common between elements with high but similar electronegativity, as is the case when nonmetals bond to each other.
- Metallic Bonding: In metallic bonding, electrons are considered to be shared collectively by a large number of atoms, forming an “electron sea” throughout the material. This type of bonding is seen when metals bond to each other, since they have similar, low electronegativity.
In the following sections of this chapter, we will examine each of these models in detail and see how the theoretical understanding explains the observable properties of materials that fall into each of these categories.
The relationship between electronegativity and bonding type in elements and binary compounds (compounds of only two elements) is illustrated by the simplified van Arkel-Ketelaar Triangle diagram shown in Figure \(\PageIndex{1}\) . Ionic bonding is shown at the point where the two elements have the largest difference in electronegativity, as in CsF. Covalent bonding is shown where the elements have low or no difference in electronegativity but a high average electronegativity, as in F2. Metallic bonding is shown where the elements have low or no difference in electronegativity and a low average electronegativity, as in Cs. These relationships can be used to predict the type of bonding likely to be exhibited by any compound based on the elements involved.
Electronegativity and Bonding Type
High Δ χ = Ionic Bonding
Low Δ χ, High χavg = Covalent Bonding
Low Δχ, Low χavg = Metallic Bonding
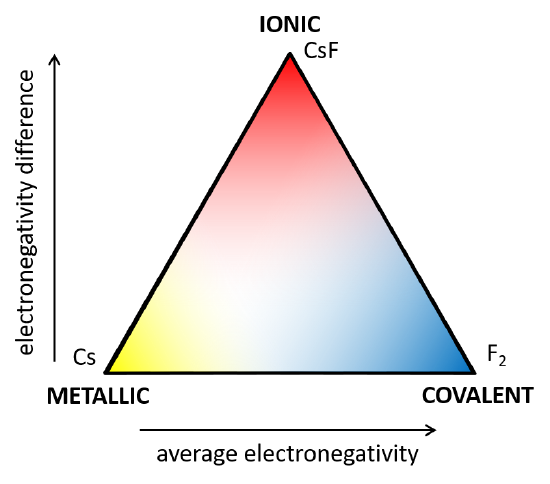
Figure \(\PageIndex{1}\). A simplified van Arkel-Ketelaar Triangle diagram showing the relationship between electronegativity and bonding type. At the extreme of high electronegativity difference is ionic bonding, exemplified by cesium fluoride. At the extreme of zero electronegativity difference and high average electronegativity is covalent bonding, exemplified by molecular fluorine. At the extreme of zero electronegativity difference and low average electronegativity is metallic bonding, exemplified by metallic cesium.
Example \(\PageIndex{1}\): Predicting Bonding Type
Predict the most likely type of bonding to describe the following compounds:
a. Bronze (alloy of Cu and Sn)
b. KBr
c. NO2
Strategy
Consider where each element in the compound falls in the periodic table and compare their electronegativity values to decide whether ionic, covalent, or metallic bonding is most likely.
Solution
a. Cu and Sn are both metals and are expected to have relatively low electronegativity values (χCu = 1.90; χSn = 1.96). Therefore, metallic bonding would be the most appropriate model.
b. K is a metal with low electronegativity (χK = 0.82), while Br is a nonmetal with relatively high electronegativity (χBr = 2.96). There is a large difference in electronegativity, suggesting that electrons will be attracted away from K toward Br. Therefore, ionic bonding would be the most appropriate model.
c. N and O are both nonmetals with high electronegativities (χN = 3.04; χO = 3.44). Therefore, covalent bonding would be the most appropriate model.
Exercise \(\PageIndex{1}\): Predicting Bonding Type
Predict the most likely type of bonding to describe the following compounds:
a. SF4
b. CaO
c. NaK
- Answer
-
a. Covalent
b. Ionic
c. Metallic
Limitations of the Models
Although the three bonding models we have described are useful for understanding the bonding of many compounds, it should be understood that they are simplifications of the reality of chemical bonding and not mutually exclusive categories. The triangle diagram in Figure \(\PageIndex{1}\) aptly illustrates that the ionic, covalent, and metallic bonding models are best regarded as extreme cases, which real compounds approach to a greater or lesser extent. Most real compounds (especially the most interesting ones!) do not fit squarely under one category of bonding, but rather have some characteristics of two or even all three.
For example, consider the compound gallium arsenide (GaAs), an important semiconductor material (Figure \(\PageIndex{2}\) ). How would we characterize its bonding? Ga is a metal with an electronegativity of about 1.8, while As is a metalloid with an electronegativity of about 2.2. Neither of their electronegativities are particularly high or particularly low, and the difference between them is also middling. If we were to situate GaAs on our diagram from Figure \(\PageIndex{1}\), it would fall somewhere in the “gray area” near the middle of the triangle rather than in one corner. In this case, it is best to describe GaAs as having bonding character of several models at once, most importantly metallic and covalent. This description is also consistent with the properties of GaAs since, as a semiconductor, it falls in between the properties of (conductive) metals and (generally nonconductive) covalent compounds.
Figure \(\PageIndex{2}\). Gallium arsenide-based solar cells covering the sides of the MidSTAR-1 satellite. Image: Public Domain from the United States Naval Academy, via Wikimedia Commons.
It is important to understand that all chemical compounds exist somewhere on this “bonding spectrum” rather than in hard-and-fast categories. We will continue to discuss compounds as being “ionic”, “covalent”, or “metallic”, but keep in mind that these are simplified descriptions of a much more complex—and fascinating—reality.
Contributors
- Anna Christianson, Bellarmine University


