5.2: Chemical Formulas
- Page ID
- 289373
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)⚙️ Learning Objectives
- Determine the number of different atoms in a formula.
- Define chemical formula, molecular formula, and empirical formula.
A chemical formula is an expression that shows the elements in a compound and the relative proportions of those elements. Water is composed of hydrogen atoms and oxygen atoms chemically bonded in a 2:1 ratio, which leads to the chemical formula for water being written as H2O.
Molecular Formula
A molecular formula is a chemical formula of a molecular compound that represents the numbers of atoms of each element present in a molecule of the compound. Ammonia is a compound of nitrogen and hydrogen as shown below:
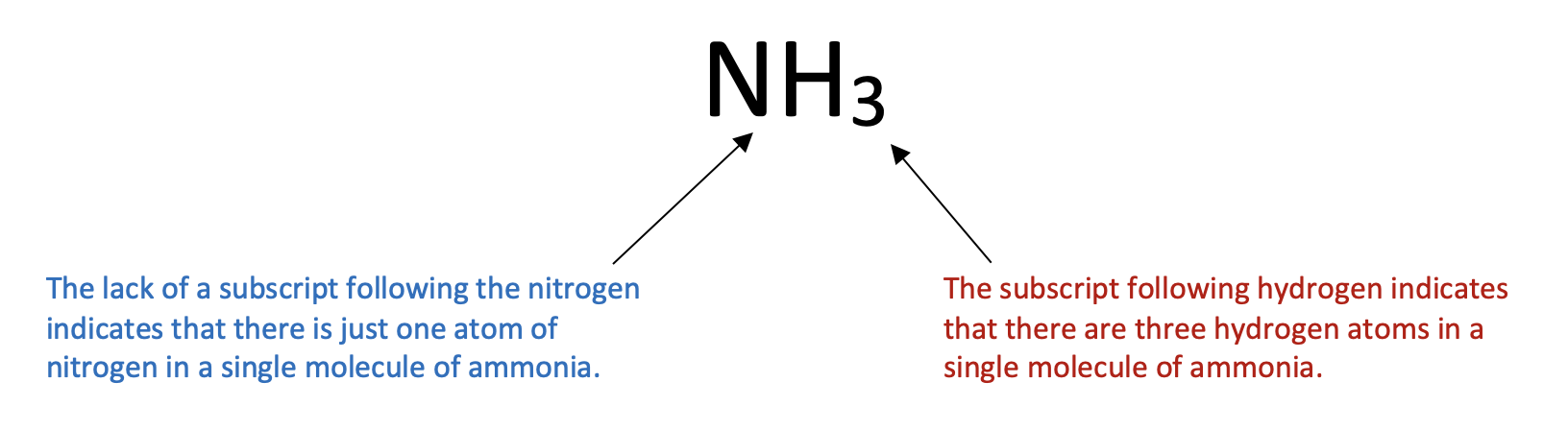
The arrangement of the elements depends on the particular structure, which is not of concern at this point. The number of atoms of each kind is indicated by a subscript following the atom. If there is more than one atom of a specific kind, the number is written as a subscript following the atom. A value of "1" is implied when there is no subscript written after an element. We would not write N3H for ammonia, because that would mean that there are three nitrogen atoms and one hydrogen atom in the molecule.
If we consider the molecular formula for sulfuric acid, H2SO4, one of the most widely produced chemicals in the United States, we could conclude that one molecule of H2SO4 contains two hydrogen atoms, one sulfur atom, and four oxygen atoms.
Empirical Formula
An empirical formula is a formula that shows the elements in a compound in their lowest whole-number ratio. Glucose is an important simple sugar that cells use as their primary source of energy. Its molecular formula is \(\ce{C_6H_{12}O_6}\). Since each of the subscripts is divisible by 6, the empirical formula for glucose is \(\ce{CH_2O}\). When chemists analyze an unknown compound, the first step is often to determine its empirical formula.
- molecular formula: C6H12O6
- empirical formula: CH2O
There are a great many compounds whose molecular and empirical formulas are the same. If the molecular formula cannot be simplified into a smaller whole-number ratio, as in the case of H2O or P2O5, then the empirical formula is also the molecular formula.
Polyatomic Ions
Certain groups of atoms often bond together to form what is called a polyatomic ion, a group that often behaves as a single unit in chemical reactions. Polyatomic ions are discussed in further detail in Section 5.7. If more than one of a particular polyatomic ion exists in a chemical formula, it will be enclosed in parentheses followed by a subscript that indicates the quantity of that polyatomic ion present in the formula. To count the total number of atoms for formulas with polyatomic ions enclosed in parentheses, the subscript is used as a multiplier for each atom or number of atoms.
✅ Example \(\PageIndex{1}\)
How many atoms of each element are represented by the formula of calcium phosphate, Ca3(PO4)2?
Solution
| Ca3(PO4)2 | Explanation |
|---|---|
| 3 Ca atoms | The subscript "3" follows the symbol of calcium. |
| 2 P atoms | P has no subscript, which implies there is 1 phosphorus atom within PO4. The subscript of "2" outside the parentheses serves as a multiplier of all the atoms within the parentheses. Therefore, 2 × 1 = 2. |
| 8 O atoms | O has a subscript of "4". The subscript of "2" outside the parentheses serves as a multiplier of all the atoms within the parentheses. Therefore, 2 × 4 = 8. |
✏️ Exercise \(\PageIndex{1}\)
Given that the blue atoms represent nitrogen and the white atoms represent hydrogen:
- What is its molecular formula? (nitrogen is written first in the formula)
- What is its empirical formula? (again, nitrogen is written first)
- Answer A
- N2H4
- Answer B
- NH2
✏️ Exercise \(\PageIndex{2}\)
How many atoms of each element are represent in each formula?
- C12H22O11
- Li2CO3
- (NH4)2Cr2O7
- Answer A
- 12 carbon, 22 hydrogen, 11 oxygen
- Answer B
- 2 lithium, 1 carbon, 3 oxygen
- Answer C
- 2 nitrogen, 8 hydrogen, 2 chromium, 7 oxygen
Summary
- A chemical formula is an expression that shows the elements in a compound and the relative proportions of those elements.
- If only one atom of a specific type is present, no subscript is used.
- For atoms that have two or more of a specific type of atom present, a subscript is written after the symbol for that atom.
- Polyatomic ions in chemical formulas are enclosed in parentheses followed by a subscript if more than one of the same type of polyatomic ion exist.
- Molecular formulas do not indicate how the atoms are arranged in the molecule.
- The empirical formula tells the lowest whole-number ratio of elements in a compound. The empirical formula does not show the actual number of atoms.
This page is shared under a CK-12 license and was authored, remixed, and/or curated by Melissa Alviar-Agnew, Lance S. Lund (Anoka-Ramsey Community College), and Henry Agnew. Original source: https://www.ck12.org/c/chemistry/.



