5.6: Ions With Variable Charges
- Page ID
- 350432
⚙️ Learning Objectives
- Write the correct names and formulas for binary ionic compounds that have ions with variable charges.
Up until now, it has been assumed that elements form ions with fixed charges. However, many metals form ions that have variable charges. In fact, the large majority of transition metals form ions with variable charges. There is also a small block of main group metals that form ions with variable charges as well (see Figure \(\PageIndex{1}\) below). As we will find out, there is no need to memorize the charges of metals that form ions with variable charges, as the charges may be identified by using Roman numerals. When writing the formula of the ion, the name of the metal is immediately followed by parentheses (no space) within which is a Roman numeral equal to the magnitude of charge on the ion.
For example,
- the iron(II) ion has a formula of Fe2+.
- the iron(III) ion has a formula of Fe3+.
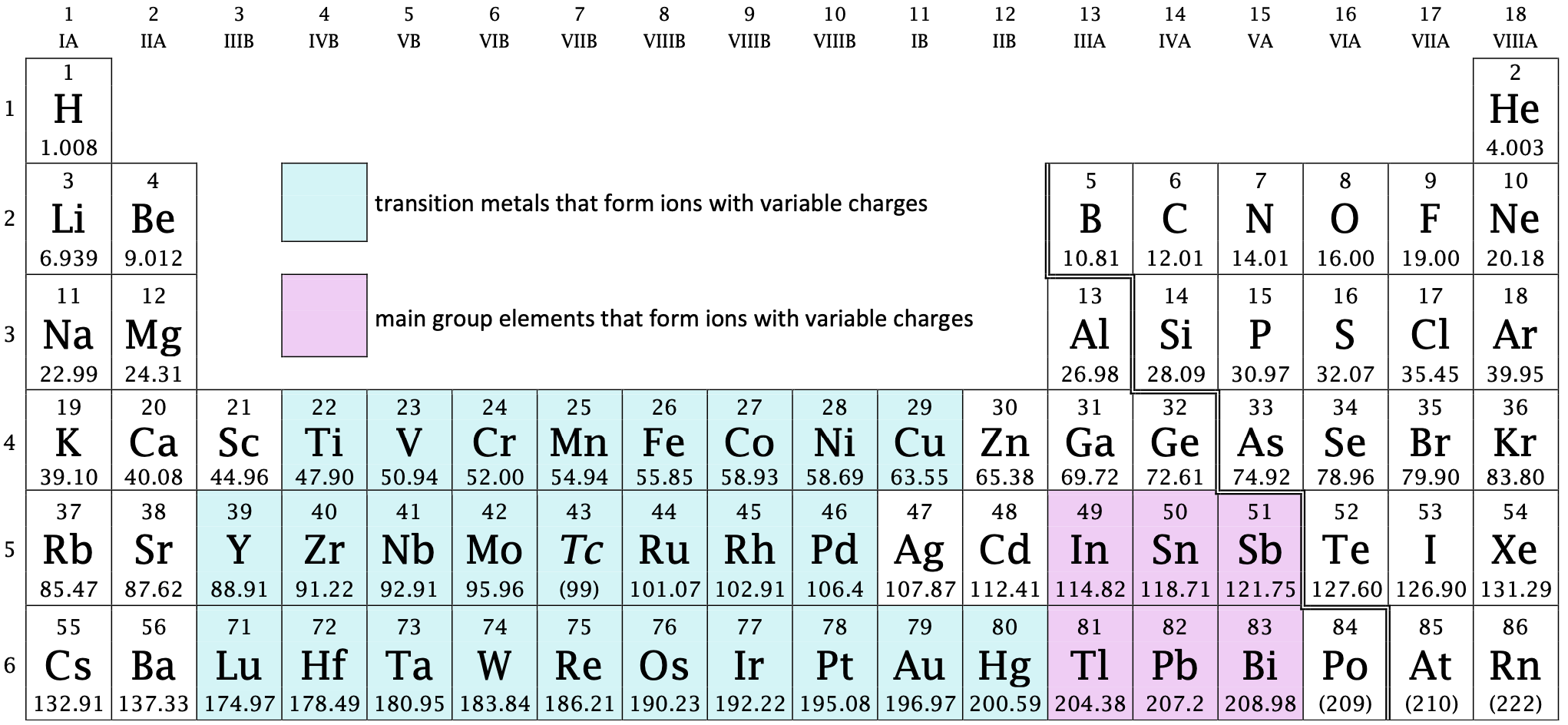
Figure \(\PageIndex{1}\): The periodic table showing elements that form ions with variable charges.
✏️ Exercise \(\PageIndex{1}\)
If the formula of an ion is provided, write the name. If the name of an ion is provided, write the formula.
- Mn3+
- copper(I)
- lead(IV)
- Cu2+
- Answer A
- manganese(III)
- Answer B
- Cu+
- Answer C
- Pb4+
- Answer D
- copper(II)
When writing names for compounds containing metals that form ions with variable charges, the name must include the Roman numeral that shows the charge on the metal ion. Roman numerals are not included when the metal only forms ions with fixed charges. Chemical formulas themselves will never include Roman numerals as the charge on the metal ions present may be determined from the subscripts in the chemical formula.
✅ Example \(\PageIndex{1}\): Writing Chemical Formulas
Write the chemical formula for each compound.
- iron(II) oxide
- iron(III) oxide
Solution
| iron(II) oxide | iron(III) oxide | ||
|---|---|---|---|
|
Fe2+ O2– | Fe3+ O2– | |
|
Fe2+ O2– | Fe3+ Fe3+ |
O2– O2– O2– |
|
2+ balances 2– | 6+ balances 6– | |
|
FeO | Fe2O3 | |
✅ Example \(\PageIndex{2}\): Writing Chemical Names
Give the correct name for each compound.
- CuO
- Cu2O
Solution
| CuO | Cu2O | ||
|---|---|---|---|
|
copper( ) oxide | copper( ) oxide | |
|
Cu?+ O2– | Cu?+ Cu?+ |
O2– |
|
Cu?+ must have a charge of 2+ (Cu2+) to balance oxide's charge of 2– (O2–). | Each Cu?+ must have a charge of 1+ (Cu+) to balance oxide's charge of 2– (O2–). | |
|
Cu2+ means copper(II), so the name is: copper(II) oxide |
Cu+ means copper(I), so the name is: copper(I) oxide |
|
✏️ Exercise \(\PageIndex{2}\)
If a formula is provided, write the name of the compound. If a name is provided, write the formula of the compound.
- titanium(IV) sulfide
- Co2O3
- PbCl2
- Answer A
- TiS2
- Answer B
- cobalt(III) oxide
- Answer C
- lead(II) chloride
Stock System vs. Common Names
The approach for naming the ions for metals that have variable charges is called the Stock system, in which an ion’s positive charge is indicated by a Roman numeral in parentheses after the element name. The Na+ ion is not called the sodium(I) ion because (I) is unnecessary. Sodium forms only a 1+ ion, so there is no ambiguity about the name sodium ion.
A second system, called the common system, is not conventional but is still prevalent and used in the health sciences. This system recognizes that many metals have two common cations. The common system uses two suffixes (-ic and -ous) that are appended to the root name of the element. The -ic suffix represents the cation with the larger of the two charges, and the -ous suffix represents the cation with the smaller of the two charges. In many cases, the stem of the element name comes from the Latin name of the element. Table \(\PageIndex{1}\) lists the elements that use the common system, along with their respective cation names. This text uses the Stock system of nomenclature.
This page is shared under a CK-12 license and was authored, remixed, and/or curated by Lance S. Lund (Anoka-Ramsey Community College), Melissa Alviar-Agnew, and Henry Agnew. Original source: https://www.ck12.org/c/chemistry/.



