1.10: Metric Prefixes
- Page ID
- 434747
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe how prefixes are used in the metric system.
- Identify how the metric prefixes nano, micro, milli-, centi-, deci, and kilo- compare to the base unit.
Many aspects of chemistry use quantitative measurements to describe properties of matter. In this section, we will look at how we deal with very large or very small measured numbers, with prefixes that are used with units of measurements.
Metric Prefixes
Conversions between metric system units are straightforward because the system is based on powers of ten. For example, meters, centimeters, and millimeters are all metric units of length. There are 10 millimeters in 1 centimeter and 100 centimeters in 1 meter. Metric prefixes are used to distinguish between units of different size. These prefixes all derive from either Latin or Greek terms. For example, mega comes from the Greek word \(\mu \varepsilon \gamma \alpha \varsigma\), meaning "great". Table \(\PageIndex{1}\) lists the most common metric prefixes and their relationship to the central unit that has no prefix. Length is used as an example to demonstrate the relative size of each prefixed unit.
| Prefix | Unit Abbreviation | Meaning | Example |
|---|---|---|---|
| giga | \(\text{G}\) | 1,000,000,000 | 1 gigameter \(\left( \text{Gm} \right)=10^9 \: \text{m}\) |
| mega | \(\text{M}\) | 1,000,000 | 1 megameter \(\left( \text{Mm} \right)=10^6 \: \text{m}\) |
| kilo | \(\text{k}\) | 1,000 | 1 kilometer \(\left( \text{km} \right)=1,000 \: \text{m}\) |
| hecto | \(\text{h}\) | 100 | 1 hectometer \(\left( \text{hm} \right)=100 \: \text{m}\) |
| deka | \(\text{da}\) | 10 | 1 dekameter \(\left( \text{dam} \right)=10 \: \text{m}\) |
| 1 | 1 meter \(\left( \text{m} \right)\) | ||
| deci | \(\text{d}\) | 1/10 | 1 decimeter \(\left( \text{dm} \right)=0.1 \: \text{m}\) |
| centi | \(\text{c}\) | 1/100 | 1 centimeter \(\left( \text{cm} \right)=0.01 \: \text{m}\) |
| milli | \(\text{m}\) | 1/1,000 | 1 millimeter \(\left( \text{mm} \right)=0.001 \: \text{m}\) |
| micro | \(\mu\) (mc) | 1/1,000,000 | 1 micrometer \(\left( \mu \text{m} \right)=10^{-6} \: \text{m}\) |
| nano | \(\text{n}\) | 1/1,000,000,000 | 1 nanometer \(\left( \text{nm} \right)=10^{-9} \: \text{m}\) |
| pico | \(\text{p}\) | 1/1,000,000,000,000 | 1 picometer \(\left( \text{pm} \right)=10^{-12} \: \text{m}\) |
The abbreviation for metric prefix micro is mc in the field of medicine.
There are a couple of odd little practices with the use of metric abbreviations. Most abbreviations are lowercase. We use "\(\text{m}\)" for meter and not "\(\text{M}\)". However, when it comes to volume, the base unit "liter" is abbreviated as "\(\text{L}\)" and not "\(\text{l}\)". So we would write 3.5 milliliters as \(3.5 \: \text{mL}\).
As a practical matter, whenever possible you should express the units in a small and manageable number. If you are measuring the weight of a material that weighs \(6.5 \: \text{kg}\), this is easier than saying it weighs \(6500 \: \text{g}\) or \(0.65 \: \text{dag}\). All three are correct, but the \(\text{kg}\) units in this case make for a small and easily managed number. However, if a specific problem needs grams instead of kilograms, go with the grams for consistency.
Give the abbreviation for each unit and define the abbreviation in terms of the base unit.
- kiloliter
- microsecond
- decimeter
- nanogram
Solutions
| Explanation | Answer | |
|---|---|---|
| a | The prefix kilo means “1,000 ×,” so 1 kL equals 1,000 L. | kL |
| b | The prefix micro implies 1/1,000,000th of a unit, so 1 µs (mcs) equals 0.000001 s. | mcs |
| c | The prefix deci means 1/10th, so 1 dm equals 0.1 m. | dm |
| d | The prefix nano means 1/1000000000, so a nanogram is equal to 0.000000001 g. | ng |
Give the abbreviation for each unit and define the abbreviation in terms of the base unit.
- kilometer
- milligram
- nanosecond
- centiliter
- Answer a:
- km
- Answer b:
- mg
- Answer c:
- ns
- Answer d:
- cL
Convert 81.2 g to kilograms following using the dimensional analysis. Show clearly conversion factors.
Solution
1 kg = 103 g
Two conversion factors are possible:
 and
and 
Use the first conversion factor to cancel out the units of grams:

Problem Solving With Multiple Conversions
Sometimes you will have to perform more than one conversion to obtain the desired unit. For example, suppose you want to convert 54.7 km into millimeters. Kilo and milli are both metric prefixes. You can do the conversion in two steps.
To convert one metric prefix to another, it is useful to go through the base unit. We will set up a series of conversion factors so that each conversion factor produces the next unit in the sequence. We first convert the given amount in km to the base unit which is meters. We know that 1 km = 1,000 m.
\[ 54.7\; \cancel{\rm{km}} \times \dfrac{1,000 \; \rm{m}}{1\; \cancel{\rm{km}}} = 54,700\; \rm{m} \nonumber \]
Then we convert meters to mm, remembering that 1 mm = 10−3 m.
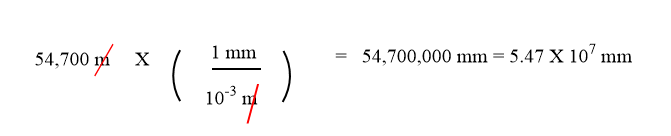
We have expressed the final answer in scientific notation.
As a shortcut, both steps in the conversion can be combined into a single, multistep expression:
Concept Map
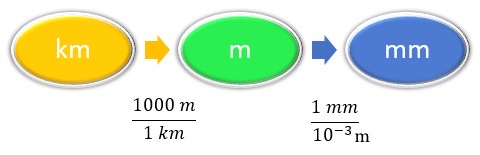
Calculation

In each step, the previous unit is canceled and the next unit in the sequence is produced, each successive unit canceling out until only the unit needed in the answer is left.
Either method—one step at a time or all the steps together—is acceptable. If you do all the steps together, the restriction for the proper number of significant figures should be done after the last step. As long as the math is performed correctly, you should get the same answer no matter which method you use.
Convert 58.2 milliseconds (ms) to megaseconds (Ms) in one multi-step calculation.
Solution
|
Steps for Problem Solving |
Unit Conversion |
|---|---|
|
Identify the "given" information and what the problem is asking you to "find." |
Given: 58.2 ms Find: Ms |
| List other known quantities |
1 ms = 10−3 s 1 Ms = 106 s |
|
Prepare a concept map |
 |
|
Calculate |
Neither conversion factor affects the number of significant figures in the final answer. |
Convert 43.007 mg to kilograms in one multistep calculation.
- Answer
-
\[ \begin{align*} 43.007 \; \cancel{\rm{mg}} \times \dfrac{\cancel{1 \rm{g}}}{1,000\; \cancel{\rm{mg}}} \times \dfrac{1\; \rm{kg}}{1,000\; \cancel{ \rm{g}}} &=0.000043007\; \rm{kg} \\[4pt] &= 4.3007 \times 10^{-5}\; \rm{kg} \end{align*} \nonumber \].
Neither conversion factor affects the number of significant figures in the final answer.
Write a concept map (a plan) for how you would convert \(1.0 \times 10^{12}\) nanoliters (nL) to kiloliters (kL).
Solution
Concept Map: Convert the given (nanoliters, nL) to liters; then convert liters to kiloliters.

Summary
Metric prefixes derive from Latin or Greek terms. The prefixes are used to make the measured numbers manageable. The SI system is based on multiples of ten.