3.2.1: Elementary Reactions
- Page ID
- 1410
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)An elementary reaction is a single step reaction with a single transition state and no intermediates.
Introduction
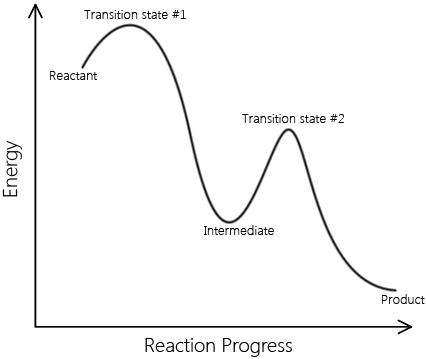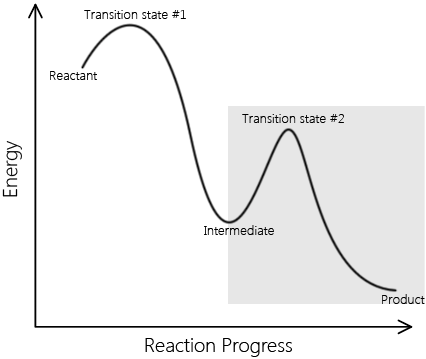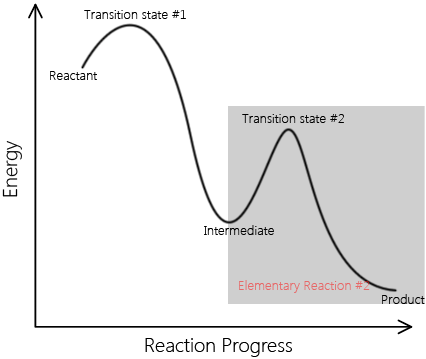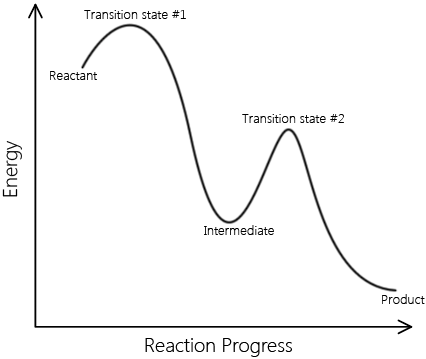
Elementary reactions add up to complex reactions; non-elementary reactions can be described by multiple elementary reaction steps. A set of elementary reactions comprises a reaction mechanism, which predicts the elementary steps involved in a complex reaction. Below are two reaction coordinates of two reactions. One describes an elementary reaction, and the other describes a non-elementary reaction.
| Elementary Reaction (one step) | Two Step Reaction |
|
\[ \text{Reactants} \rightarrow \text{Products} \nonumber \] This is a sample reaction coordinate of an elementary reaction. Note that there is one transition state and no intermediate. Elementary steps cannot be broken down into simpler reactions. |
\[ \text{Reactants} \rightarrow \text{Intermediates} \rightarrow \text{Products} \nonumber \]
This is a sample reaction coordinate of a complex reaction. Note that it involves an intermediate and multiple transition A complex reaction can be explained in terms of elementary reactions. |
Types of Elementary Reactions
The molecularity of a reaction refers to the number of reactant particles involved in the reaction. Because there can only be discrete numbers of particles, the molecularity must take an integer value. Molecularity can be described as unimolecular, bimolecular, or termolecular. There are no known elementary reactions involving four or more molecules.1
 |
 |
 |
| Unimolecular Reaction |
Bimolecular Reaction |
Termolecular Reaction |
Unimolecular Reaction
A unimolecular reaction occurs when a molecule rearranges itself to produce one or more products. An example of this is radioactive decay, in which particles are emitted from an atom. Other examples include cis-trans isomerization, thermal decomposition, ring opening, and racemization. The rate at which a substance decomposes is dependent on its concentration. Unimolecular reactions are often first-order reactions as explained by Frederick Alexander Lindemann, which is referred to as the Lindemann mechanism.
Bimolecular Reaction
A bimolecular reaction involves the collision of two particles. Bimolecular reactions are common in organic reactions such as nucleophilic substitution. The rate of reaction depends on the product of the concentrations of both species involved, which makes bimolecular reactions second-order reactions.
Termolecular Reaction
A termolecular reaction requires the collision of three particles at the same place and time. This type of reaction is very uncommon because all three reactants must simultaneously collide with each other, with sufficient energy and correct orientation, to produce a reaction. There are three ways termolecular reactions can react, and all are third order.
| Molecularity | Elementary Step | Rate Law | Example |
|---|---|---|---|
| Unimolecular | \(A \rightarrow Products\) | \(rate = k[A]\) | \(N_2O_{4(g)} \rightarrow 2NO_{2(g)}\) |
| Bimolecular | \(A + A \rightarrow Products\) | \(rate = k[A]^2\) | \(2NOCl \rightarrow 2NO_{(g)} + Cl_{2(g)}\) |
| \(A + B \rightarrow Products\) | \(rate = k[A][B]\) | \(CO_{(g)} + NO_{3(g)} \rightarrow NO_{2(g)} + CO_{2(g)}\) | |
| Termolecular | \(A + A +A \rightarrow Products\) | \(rate = k[A]^3\) | |
| \(A + A + B \rightarrow Products\) | \(rate = k[A]^2[B]\) | \(2NO_{(g)} + O_{2(g)} \rightarrow 2NO_{2(g)}\) 2 | |
| \(A + B + C \rightarrow Products\) | \(rate = k[A][B][C]\) | \(H + O_{2(g)} + M \rightarrow HO_{2(g)} + M\) 3 |
Practice Problems
1. How are non-elementary steps and elementary steps related? 2. Choose the correct statements.
3. Which of the following elementrary reactions is a termolecular reaction?
4. Which rate law corresponds to a bimolecular reaction?
5. Give an example of a reaction with a molecularity of 1/2. 6. True or False: Given species A and B inside a container, instruments detect that three (3) collisions occured before product was formed. That is, we know a reaction occured after detecting three collisions in a box. We can conclude that the reaction is a termolecular reaction (as the reaction could have been produced from A+A+B or A+B+B).
Solutions
References
- Chang, Raymond. "Chemical Kinetics." Physical Chemistry for the Biosciences. Sansalito, CA: University Science, 2005. 325-328. Print.
- Olbregts, J. (1985), Termolecular reaction of nitrogen monoxide and oxygen: A still unsolved problem. International Journal of Chemical Kinetics, 17: 835–848. doi: 10.1002/kin.550170805.
- Kerr, James Alistair. "Notes on the Tables." CRC Handbook of bimolecular and termolelucar gas reactions . Boca Raton, Florida: CRC Press, 1987. 2. Print.
- Baer, Tomas, and William L. Hase. "Introduction." Unimolecular reaction dynamics: theory and experiments. New York: Oxford University Press, 1996. 4-5. Print.
Contributors and Attributions
- Tho Nguyen (UCD), Minh Ngo (UCD)