Beckmann Rearrangement
- Page ID
- 15606
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Introduction
The Beckmann rearrangement is a reaction employed in many sectors to convert oximes to amides. The reaction has greatly been improved since its discovery in the sense of safety and viability. This work focuses on the history of the Beckmann rearrangement and improvements applied to current syntheses of mass-produced, widely available compounds that previously utilized expensive, toxic, and difficult to synthesize or hard to obtain reagents.
The Beckmann rearrangement is a reaction discovered in the mid-1880’s by the chemist Ernst Otto Beckmann. The reaction converts oximes into their corresponding amides1 allowing the insertion of the nitrogen atom from the C=N bond into the carbon chain forming a C–N bond. Depending on the starting material, it could also produce nitriles from aldehydes.2 Traditional methods for the rearrangement involve harsh reaction conditions, such as a strongly acidic medium and high temperatures that can lead to undesired side products and unsuitable for sensitive substrates.3 Further historical accounts on the reaction’s discovery shall be discussed shortly, along with the current advancements for reactions that employ the Beckmann rearrangement for mass-produced compounds in the chemical, pharmaceutical, and agricultural sectors
General Reaction
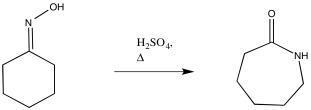
Mechanism
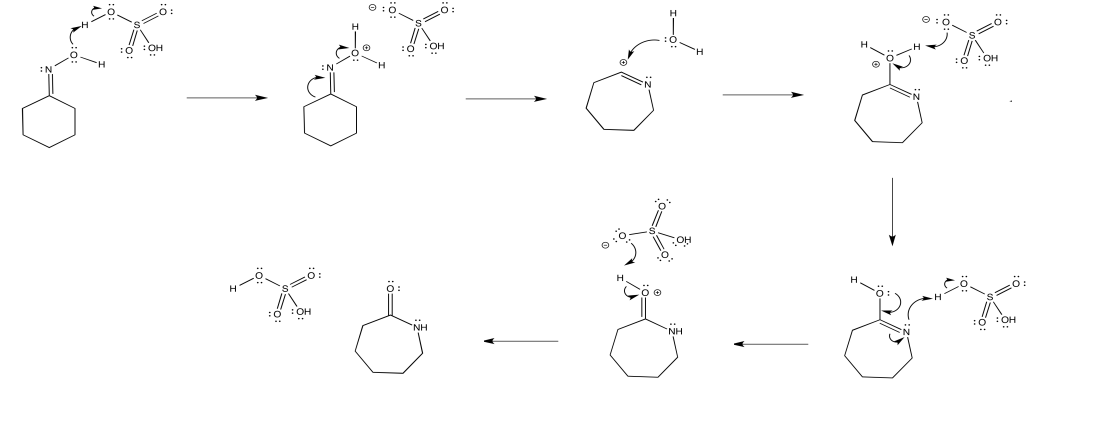
In 1883, a chemist by the name of Alois Janny was working on the reaction of acetoxime with phosphorus pentachloride but could not identify the products.6 It was not until 1886, when Beckmann published his work identifying the products that were being produced by a ketoxime (benzophenone oxime) reacting with phosphorus pentachloride or phosphorus oxychloride, and determined that the product of his reaction was benzanilide.1 Originally, Beckmann did not intend to identify the products that Janny failed to. Beckmann was working on a method to distinguish between aldehydes and ketones.1 Just like many other discoveries, Beckmann’s was predominantly accidental. Beckmann determined as well that a ketoxime could be reacted with other reagents, such as sulfuric acid.1,7 Beckmann presented his so-called “Beckmann mixture” which consisted of hydrochloric acid, acetic anhydride, and acetic acid.8 His traditional method calls for a highly acidic medium and high temperatures. The highly acidic medium and high temperatures are what can be considered harsh, as these conditions are not suitable for sensitive substrates. Methods employed today vary from “one-pot” reactions to a few step reactions, low-to-mild temperatures, have low reaction times, and use readily available, less toxic, cost-effective reagents. Within the last 25 years, green chemistry studies have focused on employing these methods in effort to minimize the cost of production, waste, and use of toxic reagents.
In 1900, Otto Wallach discovered the compound caprolactam, and the commercial synthesis consists of the acid-catalyzed Beckmann rearrangement of cyclohexanone oxime.9 Today, many reagents and methods can lead to the synthesis of caprolactam. Some reactions employ Ga(OTf)3, HgCl2, and P2O5 or Eaton's reagent (7.7% P2O5 in methanesulfonic acid) as catalysts. The Ga(OTf)3 and HgCl2 catalysts were put in a CH3CN mixture with the cyclohexanone oxime. The mixture utilizing Ga(OTf)3 was reacted at 40ºC for 20 min, which led to a 92% conversion.10 The mixture with HgCl2 was heated at 80ºC and allowed to react for 8 h.11 The mixture utilizing P2O5 or Eaton’s reagent used an ionic liquid, bmiPF6 (1-n-butyl-3-methylimidazoliumhexafluorophosphate). The optimum temperature for the P2O5 or Eaton’s reagent mixture was 75ºC, and ran for 16 h and 21 h, respectively.12
Paracetamol was discovered in the late 1800s and its use as a drug did not come about until the mid-1900s. Celanese Corp., USA patented their synthesis of paracetamol in 1985. Their work states that paracetamol is produced by reacting a hydroxy aromatic ketone (4-hydroxyacetophenone) with a hydroxylamine salt to form the ketoxime and exposing the ketoxime to a catalyst which induces the Beckmann rearrangement forming the N-acyl-hydroxy aromatic amine. For the Beckmann rearrangement to take place, 4-hydroxyacetophenone oxime was allowed to react in a mixture of Amberlyst 15 (catalytic resin) and acetic acid, which was then refluxed under N2 for 2 h, resulting in a good yield (66.7%).14 The Beckmann rearrangement was utilized for its synthesis more recently by reacting 4-hydroxyacetophenone oxime with ammonium persulphate and dimethyl sulfoxide in 1,4-dioxane. This mixture was heated to 100ºC and allowed to react 45 min, and results in a good yield.15
Contributors and Attributions
- Jorge Calderon Moreno, Department of Chemistry, Sonoma State University
References
[1] E. Beckmann, Ber. Dtsch. Chem. Ges. 1886, 19, 988-993.
[2] A. Martínez-Asencio, M. Yus, D. J.Ramon, Tetrahedron, 2012, 68, 3948-3951.
[3] S. Srivastava and K. Kaur, New J. Chem., 2020, 3.
[4] B. Waskow, et al., Tetrahedron Lett., 2016, 57, 5575-5580.
[5] Beckmann Rearrangement https://www.alfa.com/en/beckmann-rearrangement/ (accessed April 20, 2021)
[6] A. Janny, Ber. Dtsch. Chem. Ges., 1883, 16, 172.
[7] E. Beckmann, Ber. Dtsch. Chem. Ges., 1887, 20, 1507.
[8] E. Beckmann, Ber. Dtsch. Chem. Ges., 1887, 20, 2580-2585.
[9] Molecule of the Week Archive Caprolactam, https://www.acs.org/content/acs/en/molecule-of-the-week/archive/c/caprolactam.html (accessed Mar 30, 2021).
[10] P. Yan, P. Batamack, G. K. S. Prakash and G. A. Olah, Catal. Lett., 2005, 103, 165-168.
[11] C. Ramalingan and Y. -T. Park, J. Org. Chem., 2007, 72, 4536-4538.
[12] R. X. Ren, L. D. Zueva, W. Ou, Tetrahedron Lett., 2001, 42, 8441-8443.
[13] Acetominophen, https://.ncbi.nlm.nih.gov/compound/A...inophenpubchem (accessed May 11, 2021).
[14] K. G. Davenport, C. B. Hilton, Process For Producing N-acyl-hydroxy Aromatic Amines,
https://worldwide.espacenet.com/pate...n%3DUS4524217A (accessed Apr 18, 2021).
[15] S. B. Mhaske, P. S. Mahajan, A Process For Synthesis Of Amides Via Radical-mediated Beckmann Rearrangement, 2016, 1-22.

