1.5: Coordination Polymers
- Page ID
- 190561
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Polymers are long-chain molecules formed from individual molecular building blocks. Typically, the building blocks are organic molecules held together via covalent bonds. What other kinds of building blocks are available?
The formation of coordination compounds is one of many important aspects of inorganic chemistry. In a coordination compound, an electron pair donor, called a ligand, shares its electron pair with a metal atom. Frequently, the metal atom is a transition metal, and very commonly it is a transition metal ion, but there are other examples as well.
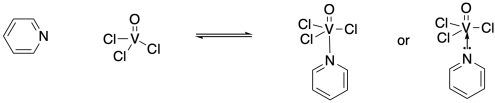
For example, the nitrogen in a pyridine ring has a lone pair. Pyridine can act as a ligand if its lone pair is shared with a metal center, such as the vanadium in trichloro(oxo)vanadium. The lone pair becomes a nitrogen-vanadium bond. Sometimes, this bond is drawn as a short arrow from the lone pair to the vanadium, emphasizing its origin, but more often it is simply drawn as a line, like any other bond.
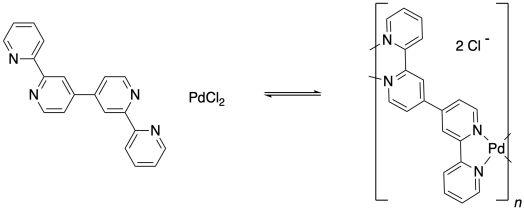
One of the interesting things about metal atoms is their capacity to form variable numbers of bonds. Although palladium dichloride could form a coordination complex by binding with one pyridine ligand, it can also do so with two pyridines. In the former case, it would form a three-coordinate complex, but the latter case would lead to a four-coordinate palladium compound.
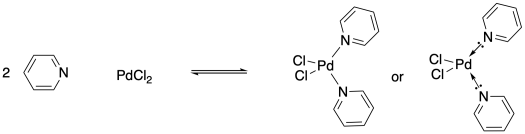
Remember, the ability to bond with two neighbouring groups, rather than just one, is an important feature that can allow a small molecule to become enchained in a polymer. The palladium here is a link in a chain that is three units long. What if the ligand also had this capacity to bind to two things? What if, instead of pyridine, the ligand were 4,4'-bipyridine?
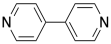
That molecule could bind a palladium atom on either end. Once a bpy (that's an abbreviation for bipyridine, pronounced "bippy") bound to a palladium atom, it would still have a second nitrogen lone pair that it could use to bind another. The palladium, too, would be free to bind a second bpy. As a result, these two monomeric units are able to form an alternating chain.
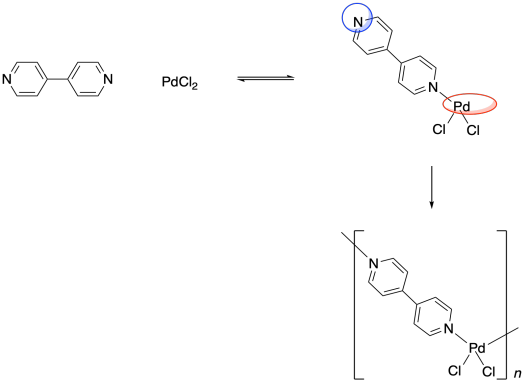
Why would people want to do that? The general idea is to exploit the properties of these metal atoms in new ways. What are metals good at? They are good at conducting electricity, and sometimes they hold useful magnetic properties. If these properties can be incorporated into a material that is more like organic polymers, which tend to be inexpensive and very lightweight, then maybe they can be used to make materials that will have all sorts of applications in everyday life. Metals are also very good catalysts for a wide range of reactions. A coordination polymer serves to space metal atoms out evenly within a structure that has a lot of surface area, which could promote catalytic efficiency. All of these potential uses have driven a great deal of research into coordination polymers in recent years (as well as related "metal-organic frameworks").
Now, can all of the bonds along this metal-ligand chain really hold together to form a polymer? One key difference between the covalent bonds you have seen in organic polymers and the dative bonds here is that dative bonds are reversible. There is always an equilibrium between the metal-ligand complex and the free metal and ligand. Of course, this bond might be very strong, in which case the equilibrium lies toward the metal-ligand complex. In other words, a large fraction of the material would form the metal-ligand complex. How large a fraction? The real value isn't that important at the moment. For our purposes, let's just say we put a metal and a ligand together and 90% of the molecules form a complex. And suppose that's also true in the next step, bringing another ligand into the picture to bind to the metal, and in the step after that, bringing another metal in to bind to the other end of one of the ligands, and so on. So, suppose each of these events leads to 90% product formation.
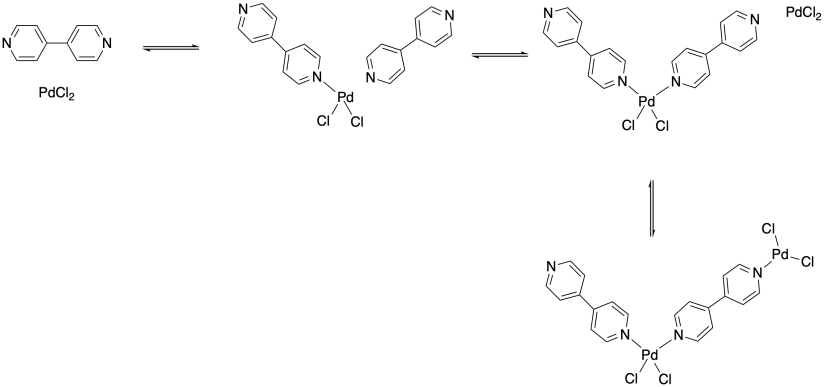
Just getting to that four-unit chain relies on three different equilibria. If, as we say, each step proceeds about 90% of the way (which sounds pretty good; you would be happy with a 90% yield on a reaction in the lab), then the entire three-step process would yield 0.90 x 0.90 x 0.90 = 0.73, or 73% product. Not bad. But useful polymer chains might be a thousand units long or more, in which case the amount of polymer actually formed of the proper chain length would be about (0.90)999 = 1.94 x 10-46 or 1.94 x 10-44 %, and that's a ridiculously small amount.
Now, a real calculation of the equilibrium concentration is of course more sophisticated than that, but this quick exercise underscores an important point: in order to form a stable coordination polymer of an appreciable length, the metal must bind the ligand very tightly. For that reason, many approaches to coordination polymers have employed multidentate ligands, which of course bind more tightly than monodentate ligands. For example, the polymer formed using the bidentate binding shown below would be much more stable with respect to depolymerization (i.e. falling apart into monomers again) than the monodentate example shown earlier.