1.2: Cyclic Carboxyloids
- Page ID
- 190558
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Carrothers' development of nylon at Du Pont led indirectly to a very similar polymer that was made in a very different way. Across the Atlantic Ocean, Paul Schlack at IG Farben in Germany was looking for a way to make a similar material that would not be subject to Du Pont's patent. His efforts led to a material called perlon, sometimes referred to as nylon 6.
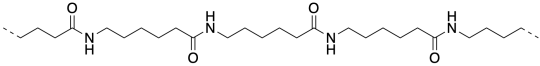
Nylon 6 is not an alternating co-polymer like nylon 66. It's just a polymer. And it isn't made from a difunctional monomer like nylon 66 or proteins. Instead, it's made from a cyclic amide, sometimes called a lactam. To polymerize, the lactam has to break open into a linear form, and the lactam monomers end up enchained head-to-tail. This process is called ring-opening polymerization.
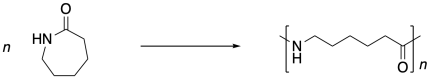
There are a number of things that are different about these two ways of making nylon. One of them turns on the whole concept of synthesis. Synthesis is the act of making things. It's a little like cooking. You gather the ingredients. You take the right amount of this and the right amount of that. You mix them together and you get something wonderful. But that's the difference here: in making nylon 66, Carrothers mixed two different compounds together. He poured one compound out of one bottle and another compound out of another bottle. He mixed them together and he got a polymer.
In contrast, Schlack didn't mix anything together, at least as far as we have seen so far. It all came out of one bottle. So why wasn't it already nylon 6 in the bottle? If the monomers just react with themselves, couldn't they have just gone ahead and done that in the bottle?
Ring-opening polymerization, at least in this context, is an example of a chain reaction. Chain reactions don't just happen by themselves; they need a jump start.
To understand why, you have to appreciate that these two materials come about through two very different classes of polymerization reactions. Nylon 66 is the product of a condensation reaction. An amine is mixed with a carboxylic acid, water is released, and an amide bond is formed. Even if the pair of reactants is chosen to be more synthetically efficient -- say, an amine and an acid chloride instead of a carboxylic acid -- a condensation reaction still results, in this case releasing hydrogen chloride.
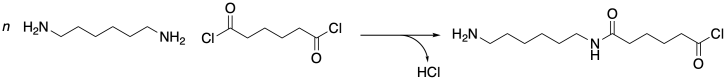
Nylon 6, on the other hand, is not the product of a condensation reaction -- at least at first glance. Look carefully at the monomer and the polymer. Count the atoms. There's nothing missing. No molecule of water or hydrogen chloride or anything else was released. The nitrogen at one end of the chain simply attaches to the carbonyl of the next.
But if you walk down to the end of that chain, until you reach the very last carbonyl, what do you find there? There's no nitrogen attached. That nitrogen was the nucleophile that bound to the carbonyl in the next monomer. There has to be something, though, because the carbon cannot be sitting there with only three bonds. So there must have been some nucleophile that added to that carbonyl, springing loose the amine that added to the next carbonyl, springing the next amine, and so on. There had to be an original nucleophile.
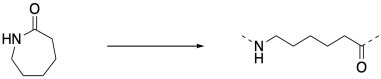
This is the jump start that the chain reaction needed. A nucleophile had to be added to get things going. Once it reacted with the first monomer, the amine became the nucleophile for the next monomer, and that ring-opening produced another amine nucleophile, and so on. Because there is always a new nucleophile produced when the next ring opens, the reaction just keeps going. That first nucleophile, the one that got everything started, is called an initiator. The need for an initiator is a hallmark of chain reactions.
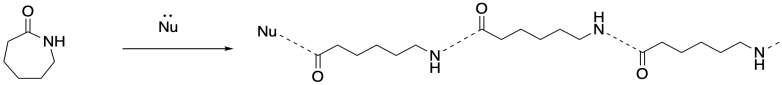
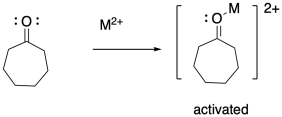
In addition to the initiator, ring-opening polymerizations frequently employ catalysts to accelerate the reaction, just as in some condensation polymerizations. The catalyst may be a Lewis acid that activates the carbonyl or an "organocatalyst" that does the same thing via hydrogen bonding. Both catalytic approaches can also make use of nucleophilic catalysis. In that case, the nucleophilicity of the nucleophile may be enhanced, or else a temporary nucleophile may add to the carbonyl until it is replaced by the nucleophilic group resulting in enchainment.
Other polyamides can also be made by ring-opening cyclic amides. The same approach is also used to prepare polyesters from cyclic esters, also called lactones. In that case, the reaction is sometimes called ring-opening trans-esterification polymerization, or ROTEP for short.


