3.5E: Initiating Crystallization
- Page ID
- 95764
At times, crystals will not form even when a solution is supersaturated, as there is a kinetic barrier to crystal formation. At times crystallization may need to be initiated, for example if the solution becomes faintly cloudy as it cools, or if the solution fails to produce crystals even when it is noticeably cooler than originally. The methods described in this section preferably should be used on solutions that are still warm, as initiating crystallization on already cool (or cold) solutions will cause too rapid a crystallization.
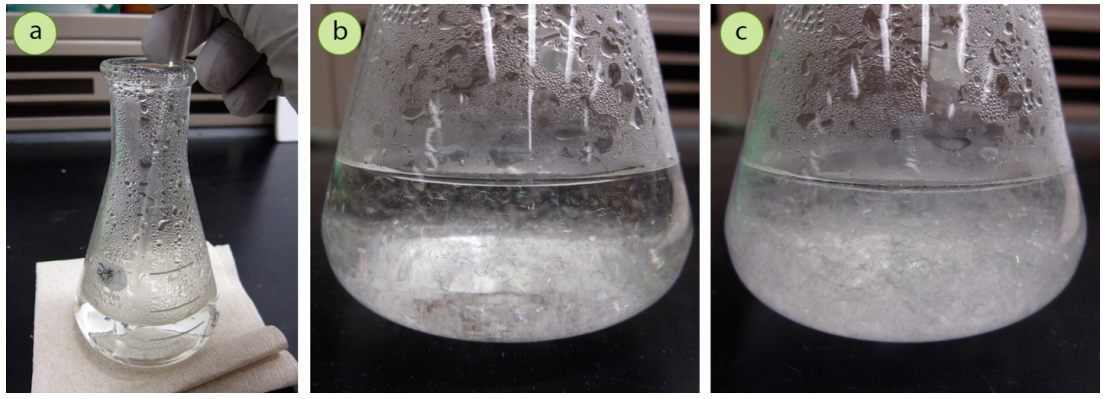
The easiest method to initiate crystallization is to scratch the bottom or side of the flask with a glass stirring rod (Figure 3.45a), with enough force that the scratching is audible (but of course not so much that you break the glass!). Crystallization often begins immediately after scratching, and lines may be visible showing crystal growth in the areas of the glass that were scratched (Figure 3.46).

Although there is no doubt that this method works, there are differences in opinion as to the mechanism of action. One theory is that scratching initiates crystallization by providing energy from the high-frequency vibrations. Another theory is that tiny fragments of glass are dislodged during scratching that provide nucleation sites for crystal formation. Another hypothesis is that the solvent may evaporate from the glass stirring rod after it is removed, and a speck of solid may fall into solution and act as a "seed crystal" (see next paragraph). It is not well understood how scratching initiates crystallization, but it is in every chemist's arsenal of "tricks".
There are a few other methods that can be used to initiate crystallization when scratching fails:
- Add a "seed crystal": a small speck of crude solid saved from before the crystallization was begun, or a bit of pure solid from a reagent jar. Seed crystals create a nucleation site where crystals can begin growth.
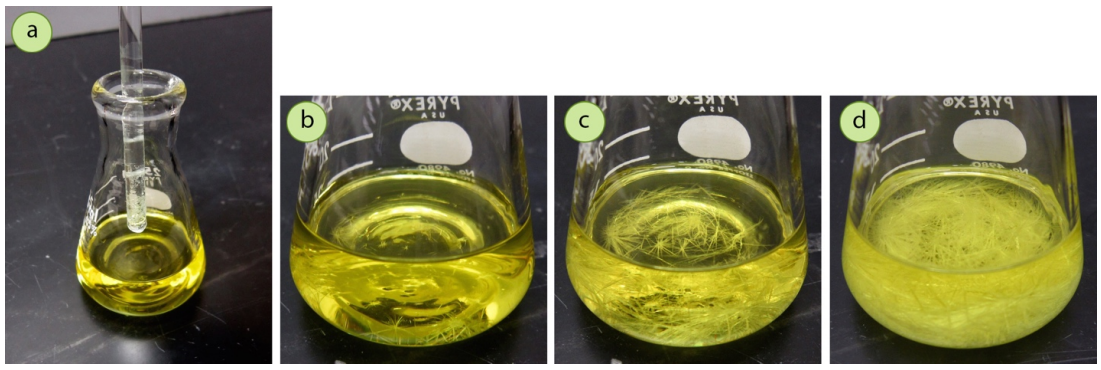
- Dip a glass stirring rod into the supersaturated solution, remove it, and allow the solvent to evaporate to produce a thin residue of crystals on the rod (Figure 3.47a). Then touch the rod to the solution's surface, or stir the solution with the rod to dislodge small seed crystals.
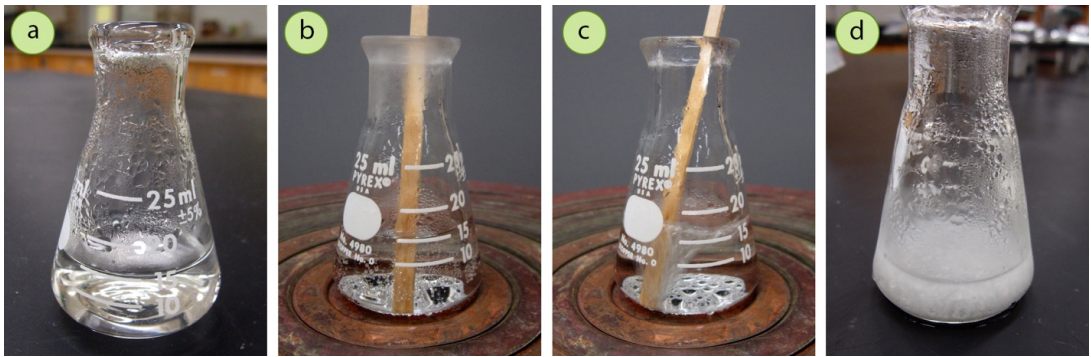
- If scratching and seed crystals do not initiate crystallization, it's possible there is too much solvent present that the compound remains completely soluble (Figure 3.48). To test if this is the case, return the solution to a boil and reduce the volume of solvent, perhaps by half (Figure 3.48c). Allow the reduced solution to cool and see if solid forms. If it does (Figure 3.48d), the quantity of solvent was definitely the problem, and solvent volumes can be experimented with to achieve the best growth of crystals.
- Use a lower temperature bath to try and encourage crystal formation. A salt water-ice bath \(\left( -10^\text{o} \text{C} \right)\) or chemical freezer are some options.


