1.3: Resonance Structures
- Page ID
- 359563
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In the case that more than one reasonable (plausible) Lewis structure can be drawn for a species, these structures are called resonance structures or resonance contributors. Resonance structures can be either equivalent or non-equivalent.
Equivalent Resonance Structures
Let’s consider the example of carbonate anion, CO32-:
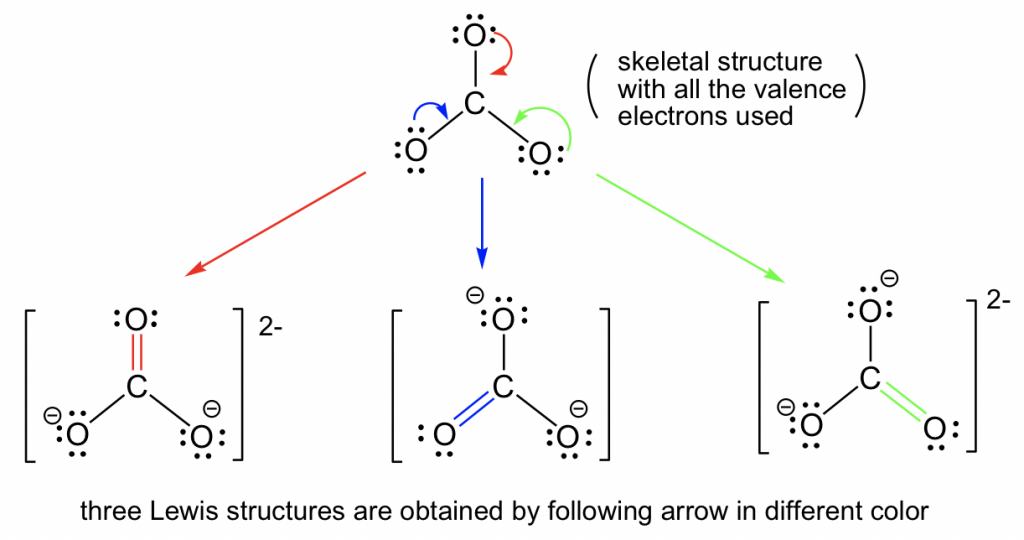
By following Step 6 in the Lewis structure drawing procedure, the double bond can be built between the central C and any of the terminal O’s to generate three structures, and they all look “the same”. However, they are not really identical (or same), they are just equivalent. Each structure is called a resonance structure, and they can be connected by the double-headed resonance arrow. There are total three equivalent resonance structures for CO32-, and the actual structure of CO32-is the hybrid of the three resonance contributors.
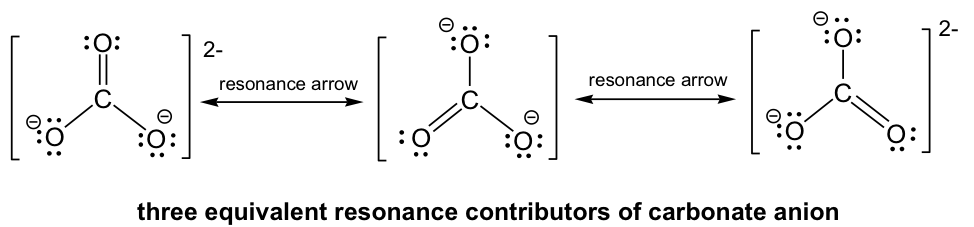
Since the resonance structures are equivalent, they are all in the same level of energy and have the same stability, so they make the same contributions to the actual structure of CO32-. This is supported by the experimental evidence that all the carbon-oxygen bonds in CO32- are the same bond length, which is longer than a regular double bond but shorter than a single bond. As a result of the resonance structures, the two negative charges in CO32- are not localized on any oxygen atoms, but are spread evenly among all three oxygen atoms, and is called charge delocalization. Because of charge delocalization, each oxygen atom has two-thirds of a full negative charge. Charge delocalization helps to stabilize the whole species. The stability a species gains from having charge delocalization through resonance contributors is called resonance stabilization effect. The greater the number of resonance contributors, the greater the resonance stabilization effect, and the more stable the species is.
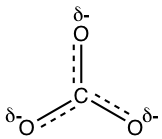
The actual structure of the carbonate anion is a combination of all the three equivalent resonance structures, that can be called a hybrid. What does the actual structure look like, and can we draw one structure on paper to show the actual structure? The actual structure can not be shown with a conventional Lewis structure, because the regular Lewis structures do not include partial charges, and there is two-thirds of a full negative charge on each oxygen atom in CO32-. An attempt to show the hybrid structure can be by using dashed lines to show that the bond between carbon and oxygen is somewhere between a single and double bond, and each oxygen atom has partial charges.
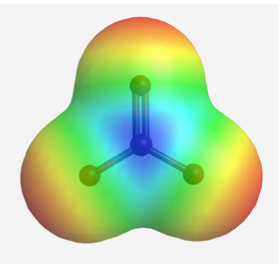
The delocalized charges can also be represented by the calculated electrostatic potential map of the electron density in the CO32- anion. In an electrostatic potential map, regions with different charges are shown in different colours. More specifically, colours trending towards red means higher negative charges, while colours trending toward blue means more positive charge (the colour system generated by different softwares might not be same, but will follow the same trend). In the electrostatic potential map of carbonate anion below, the same shade of red of all three oxygen atoms indicate the equal charge distribution at the three oxygen atoms.
Exercises 1.5
Draw all the equivalent resonance structures for following species. Include any non-zero formal charges in the structures.
- O 3 molecule
- nitrate anion NO 3 –
- chlorate anion ClO 3 – .
Answers to Practice Questions Chapter 1
Non-equivalent Resonance Structures
Resonance structures can also be non-equivalent. For the example of OCN–, there are three non-equivalent resonance structures, depending on how the multiple bonds are formed in Step 6 of the Lewis structure drawing procedure.
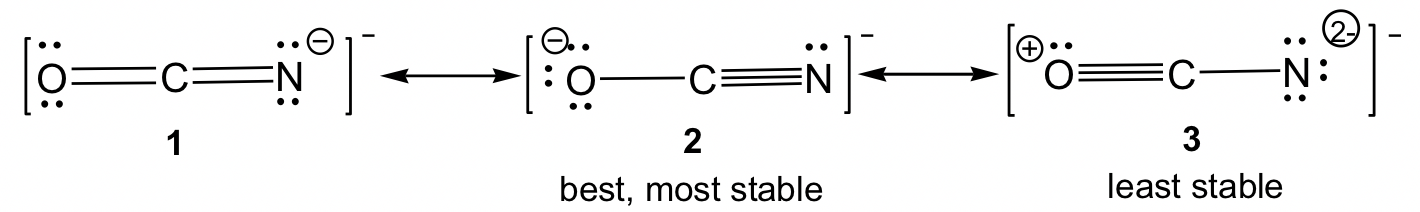
For non-equivalent resonance structures, the bonding and charge distributions are different, so they are in different energy levels. Some are more stable (better) resonance structures than others. The guidelines for comparing the relative stability between non-equivalent resonance structures are (the lower the energy, the more stable the structure is and vice versa):
- The structure with complete octets is usually more stable, except in the cases in section1.2.4 “Exceptions to Octet Rule”.
- The structure involving the smaller formal charges is more stable.
- Negative charges should be preferentially located on atoms with greater electronegativity, and positive charges should be preferentially located on atoms with less electronegativity
- Charge separation decreases the stability (increases the energy).
By applying the rules above, we can predict that for OCN–, structure 3 is the least stable one since it has the highest formal charges. For both structures 1 and 2, the formal charge is “-1”. It is more preferable for negative formal charges to be on oxygen, the more electronegative atom; therefore structure 2 is the most stable one.
Exercises 1.6
Draw all of the resonance structures for azide anion, N3–, and indicate the most stable one.


