24.8: Reactions of Arylamines
- Page ID
- 448825
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Electrophilic Aromatic Substitutions
An amino group is strongly activating and ortho- and para-directing in electrophilic aromatic substitution reactions (Section 16.4). This high reactivity of amino-substituted benzenes can be a drawback at times because it’s often difficult to prevent polysubstitution. Reaction of aniline with Br2, for instance, takes place rapidly and yields the 2,4,6-tribrominated product. The amino group is so strongly activating that it’s not possible to stop at the monobromo stage.
Another drawback to the use of amino-substituted benzenes in electrophilic aromatic substitution reactions is that Friedel–Crafts reactions are not successful (Section 16.3). The amino group forms an acid–base complex with the AlCl3 catalyst, which prevents further reaction. Both drawbacks can be overcome, however, by carrying out electrophilic aromatic substitution reactions on the corresponding amide rather than on the free amine.
As we saw in Section 21.5, treatment of an amine with acetic anhydride yields the corresponding acetyl amide, or acetamide. Although still activating and ortho-, para-directing, amido substituents () are less strongly activating and less basic than amino groups because their nitrogen lone-pair electrons are delocalized by the neighboring carbonyl group. As a result, bromination of an N-arylamide occurs cleanly to give a monobromo product, and hydrolysis of the amide with aqueous base then gives the free amine. For example, p-toluidine (4-methylaniline) can be acetylated, brominated, and hydrolyzed to yield 2-bromo-4-methylaniline. None of the 2,6-dibrominated product is obtained.
Friedel–Crafts alkylations and acylations of N-arylamides also proceed normally. For example, benzoylation of acetanilide (N-acetylaniline) under Friedel–Crafts conditions gives 4-aminobenzophenone in 80% yield after hydrolysis.
Moderating the reactivity of an amino-substituted benzene by forming an amide is a useful trick that allows many kinds of electrophilic aromatic substitutions to be carried out that would otherwise be impossible. One example is the preparation of the sulfa drugs, such as sulfanilamide.
Sulfa drugs were among the first pharmaceutical agents to be used clinically against bacterial infection. Although they have largely been replaced today by safer and more powerful antibiotics, sulfa drugs are credited with saving the lives of thousands of wounded during World War II and are still prescribed for urinary tract infections. They are prepared by chlorosulfonation of acetanilide, followed by reaction of p-(N-acetylamino)benzenesulfonyl chloride with ammonia or some other amine to give a sulfonamide. Hydrolysis of the amide then yields the sulfa drug. Note that hydrolysis of the amide can be carried out in the presence of the sulfonamide group because sulfonamides hydrolyze very slowly.
Propose a synthesis of the drug sulfathiazole from benzene and any necessary amine.
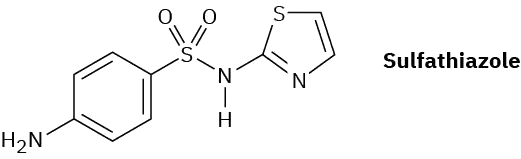
- Answer
-
1. HNO3, H2SO4; 2. H2/PtO2; 3. (CH3CO)2O; 4. HOSO2Cl; 5. aminothiazole; 6. H2O, NaOH
Propose syntheses of the following compounds from benzene:
- N,N-Dimethylaniline
- p-Chloroaniline
- m-Chloroaniline
- 2,4-Dimethylaniline
- Answer
-
- 1. HNO3, H2SO4; 2. H2/PtO2; 3. 2 CH3Br
- 1. HNO3, H2SO4; 2. H2/PtO2; 3. (CH3CO)2O; 4. Cl2; 5. H2O, NaOH
- 1. HNO3, H2SO4; 2. Cl2, FeCl3; 3. SnCl2
- 1. HNO3, H2SO4; 2. H2/PtO2; 3. (CH3CO)2O; 4. 2 CH3Cl, AlCl3; 5. H2O, NaOH
Diazonium Salts: The Sandmeyer Reaction
Primary arylamines react with nitrous acid, HNO2, to yield stable arenediazonium salts, \(\mathrm{Ar}-\stackrel{+}{\mathrm{N}} \equiv \mathrm{~N} \mathrm{X}^{-}\) a process called a diazotization reaction. Alkylamines also react with nitrous acid, but the corresponding alkanediazonium products are so reactive they can’t be isolated. Instead, they lose nitrogen instantly to yield carbocations. The analogous loss of N2 from an arenediazonium ion to yield an aryl cation is disfavored by the instability of the cation.
Arenediazonium salts are useful because the diazonio group (\(\ce{–N≡N}\)) can be replaced by a nucleophile in a substitution reaction.
Many different nucleophiles—halide, hydride, cyanide, and hydroxide among others—react with arenediazonium salts, yielding many different kinds of substituted benzenes. The overall sequence of (1) nitration, (2) reduction, (3) diazotization, and (4) nucleophilic substitution is perhaps the single most versatile method of aromatic substitution.
Aryl chlorides and bromides are prepared by reaction of an arenediazonium salt with the corresponding copper(I) halide, CuX, a process called the Sandmeyer reaction. Aryl iodides can be prepared by direct reaction with NaI without using a copper(I) salt. Yields generally fall between 60% and 80%.
Similar treatment of an arenediazonium salt with CuCN yields the nitrile ArCN, which can then be further converted into other functional groups such as carboxyl. For example, Sandmeyer reaction of o-methylbenzenediazonium bisulfate with CuCN yields o-methylbenzonitrile, which can be hydrolyzed to give o-methylbenzoic acid. This product can’t be prepared from o-xylene by the usual side-chain oxidation route because both methyl groups would be oxidized.
The diazonio group can also be replaced by –OH to yield a phenol and by –H to yield an arene. A phenol is prepared by reaction of the arenediazonium salt with copper(I) oxide in an aqueous solution of copper(II) nitrate, a reaction that is especially useful because few other general methods exist for introducing an –OH group onto an aromatic ring.
Reduction of a diazonium salt to give an arene occurs on treatment with hypophosphorous acid, H3PO2. This reaction is used primarily when there is a need for temporarily introducing an amino substituent onto a ring to take advantage of its directing effect. Suppose, for instance, that you needed to make 3,5-dibromotoluene. This product can’t be made by direct bromination of toluene because reaction would occur at positions 2 and 4. Starting with p-methylaniline (p-toluidine), however, dibromination occurs ortho to the strongly directing amino substituent, and diazotization followed by treatment with H3PO2 to remove the amino group yields the desired product.
Mechanistically, these diazonio replacement reactions occur through radical rather than polar pathways. In the presence of a copper(I) compound, for instance, it’s thought that the arenediazonium ion is first converted to an aryl radical plus copper(II), followed by subsequent reaction to give product plus regenerated copper(I) catalyst.
How would you prepare m-hydroxyacetophenone from benzene, using a diazonium replacement reaction in your scheme?

Strategy
As always, organic syntheses are planned by working retrosynthetically from the final product, one step at a time. First, identify the functional groups in the product and recall how those groups can be synthesized. m-Hydroxyacetophenone has an –OH group and a –COCH3 group in a meta relationship on a benzene ring. A hydroxyl group is generally introduced onto an aromatic ring by a four-step sequence of nitration, reduction, diazotization, and diazonio replacement. An acetyl group is introduced by a Friedel–Crafts acylation reaction.
Next, ask yourself what an immediate precursor of the target might be. Since an acetyl group is a meta director while a hydroxyl group is an ortho and para director, acetophenone might be a precursor of m-hydroxyacetophenone. Benzene, in turn, is a precursor of acetophenone.
Solution

- p-Bromobenzoic acid
- m-Bromobenzoic acid
- m-Bromochlorobenzene
- p-Methylbenzoic acid
- 1,2,4-Tribromobenzene
- Answer
-
- 1. CH3Cl, AlCl3; 2. HNO3, H2SO4; 3. SnCl2; 4. NaNO2, H2SO4; 5. CuBr; 6. KMnO4, H2O
- 1. HNO3, H2SO4; 2. Br2, FeBr3; 3. SnCl2, H3O+; 4. NaNO2, H2SO4; 5. CuCN; 6. H3O+
- 1. HNO3, H2SO4; 2. Cl2, FeCl3; 3. SnCl2; 4. NaNO2, H2SO4; 5. CuBr
- 1. CH3Cl, AlCl3; 2. HNO3, H2SO4; 3. SnCl2; 4. NaNO2, H2SO4; 5. CuCN; 6. H3O+
- 1. HNO3, H2SO4; 2. H2/PtO2; 3. (CH3CO)2O; 4. 2 Br2; 5. H2O, NaOH; 6. NaNO2, H2SO4; 7. CuBr
Diazonium Coupling Reactions
Arenediazonium salts undergo a coupling reaction with activated aromatic rings such as phenols and arylamines to yield brightly colored azo compounds, .
Diazonium coupling reactions are typical electrophilic aromatic substitutions in which the positively charged diazonium ion is the electrophile that reacts with the electron-rich ring of a phenol or arylamine. Reaction usually occurs at the para position.
Azo-coupled products are widely used as dyes for textiles because their extended conjugated \(\pi\) electron system causes them to absorb in the visible region of the electromagnetic spectrum (Section 14.9). p-(Dimethylamino)-azobenzene, for instance, is a bright yellow compound that was at one time used as a coloring agent in margarine.
Propose a synthesis of p-(dimethylamino)azobenzene with benzene as your only organic starting material.
- Answer
-
1. HNO3, H2SO4; 2. SnCl2; 3a. 2 equiv. CH3I; 3b. NaNO2, H2SO4; 4. product of 3a + product of 3b


