4.1: Introduction. Proton Exchange in Water-Acetic Acid Mixtures
- Page ID
- 366655
One of the most important applications of NMR spectroscopy is to the determination of kinetics of very fast reactions. Obviously, reaction kinetics could be followed by measuring rates of increase or decrease of resonance signals attributable to products or reactants. However, there is a very simple and much less conventional NMR method for determination of reaction rates which covers a range of rate constants and reaction types exceedingly difficult to measure in any other way.
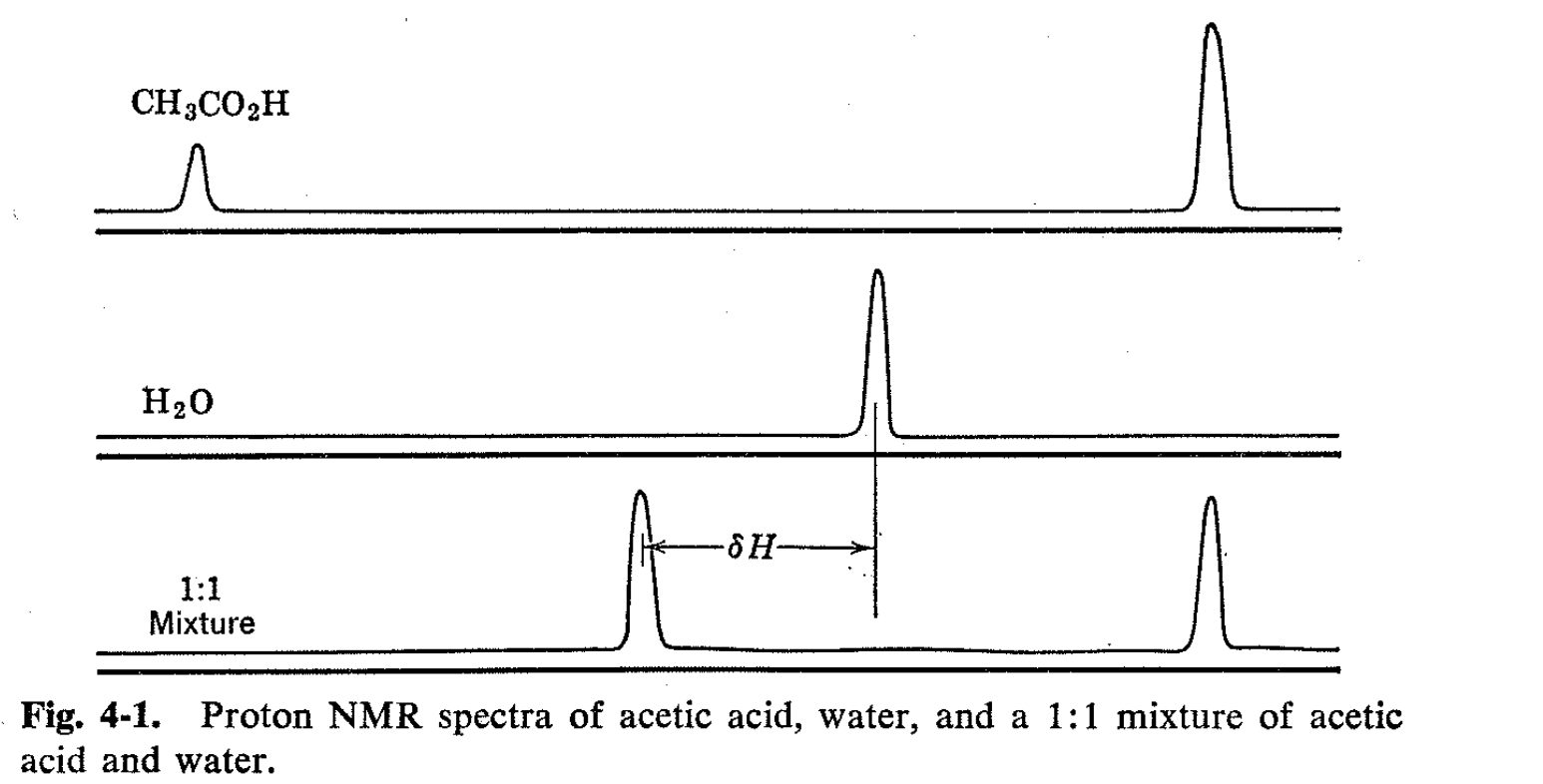
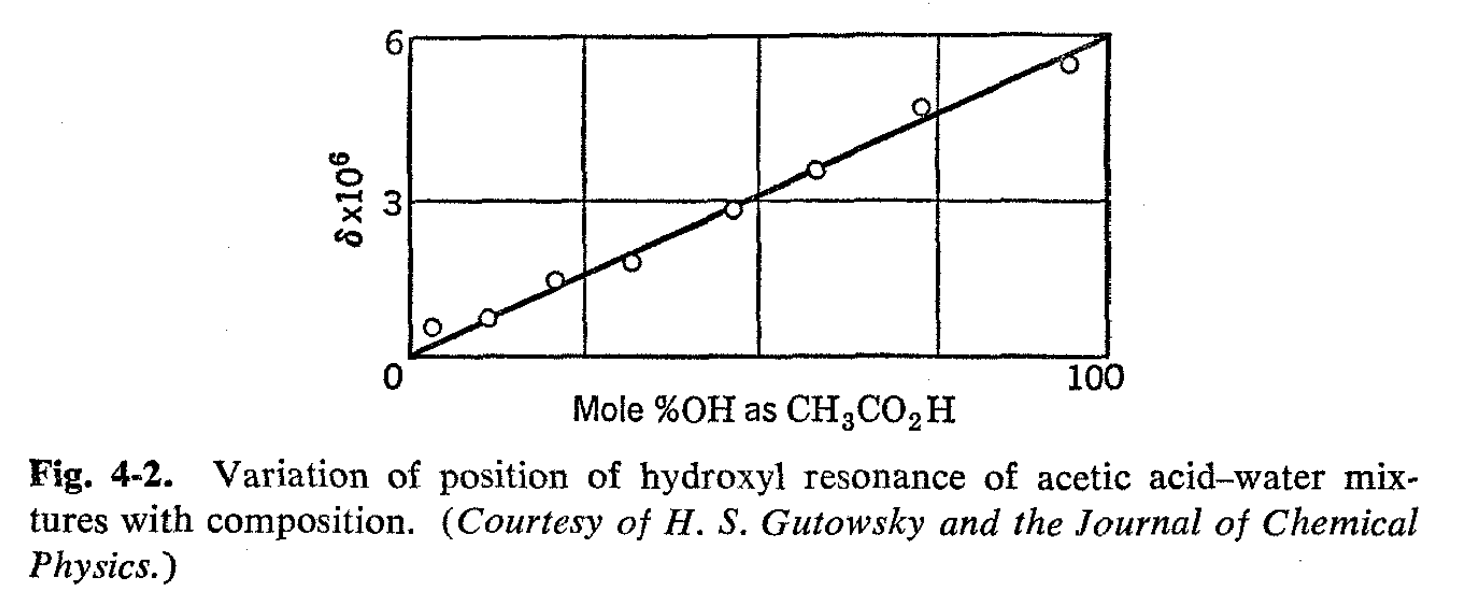
As an introduction, we consider the NMR spectra of solutions of acetic acid and water. The spectra of the two separate substances are shown in Fig. 4-1, the carboxyl hydrogen of the acetic acid appearing at a very low field compared with water and the methyl group at a considerably higher field. When the substances are mixed, the spectrum might be expected to show three lines-one for the methyl group and two others corresponding to the two varieties of hydroxyl hydrogen. This prediction is buttressed by the knowledge that a variety of physical methods show that acetic acid in water is almost completely undissociated. Nonetheless, the simple expectation is not realized. Mixtures of acetic acid and water show only two lines-a methyl resonance and a hydroxyl resonance, the latter of which is intermediate between the normal positions of the carboxyl and water proton resonances. The position of this hydroxyl resonance depends on the concentrations, and in fact, a linear relationship has been demonstrated between the line position and the fraction of the hydroxyl protons in the form of acetic acid, as shown in Fig. 4-2.1 This result is of considerable practical interest in that it provides a means of analysis by virtue of measurements of line positions rather than of line areas. This type of NMR analysis is very advantageous where applicable because line positions are far more easily and accurately measured than the integrated resonance intensities.
Apart from applications to analysis, the occurrence of an average hydroxyl resonance line position from two chemically different species of hydroxyl protons is of considerable theoretical significance. The single hydroxyl line is a consequence of rapid chemical exchange between the hydroxyl hydrogens of acetic acid and water. It represents the hydroxyl protons in a time-averaged environment. Such a phenomenon is almost unthinkable to anyone brought up exclusively on visible and ultraviolet spectroscopy. Indeed, the Franck-Condon principle states that the transition time for an electronic excitation resulting from the absorption of ultraviolet or visible electromagnetic radiation is short even compared with the rate at which the atoms vibrate in chemical bonds. However, in NMR spectroscopy, the transition times for excitation of the nuclei to higher energy magnetic states not only are long with respect to the rates of vibration of atoms in bonds but are also long with respect to rotations around single bonds and are even long with respect to rapid chemical reactions. In effect, the NMR spectrometer acts as a camera with a slow shutter speed and so records molecules in an average state taken over the relatively long periods of time required for the transitions of nuclei from one magnetic quantum state to another. The long transition times are associated with the fact that these magnetic transitions are induced by very low frequency radiations compared with the frequencies of ultraviolet and visible light.
The exchange of acetic acid and water hydroxyl protons appears always to be too fast to give anything but an average hydroxyl resonance line. With other substances, this is not always the case, and as will be seen later, separate hydroxyl absorptions can be obtained with mixtures of some hydroxyl compounds.
1 H. S. Gutowsky and A. Saika, J. Chem. Phys., 211, 1688 (1954).


