1.3: Magnetic Properties of Nuclei. Nuclear Spin
- Page ID
- 366635
Induction of an alternating magnetization in a substance like an organic compound, by an oscillatory magnetic field as described above, can be shown by isotopic substitution procedures to involve certain types of atomic nuclei which act like tiny magnets. In the ensuing discussion of magnetic properties of nuclei, we shall find it convenient to ascribe certain electromechanical properties to nuclei which are gross oversimplifications of the real state of affairs but are nonetheless very helpful in explaining how a nuclear resonance signal can arise.
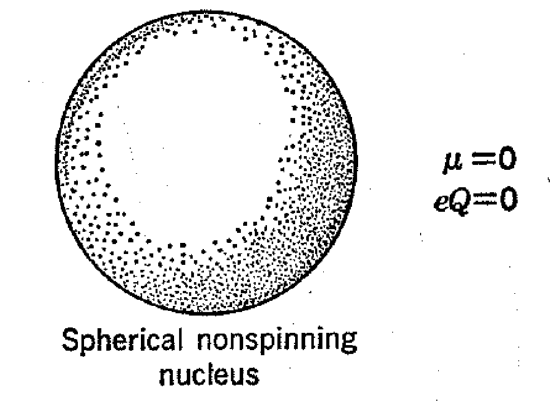
In some ways, certain nuclei behave as though they are nonspinning spherical bodies with the nuclear charge distributed evenly over their surfaces. This type of nucleus does not have a magnetic moment because there is no circulation of the nuclear charge. We also say that the "nuclear quadrupole moment" is zero because, when a probing electrical charge approaches such a nucleus, it experiences an electrostatic field, the magnitude of which is independent of the direction of approach. These nuclei are said to have their "nuclear spin" value equal to zero and, not having a magnetic moment, they can give no nuclear resonance signal. Many nuclei of considerable importance to organic chemistry, particularly 12C and 16O, are of this type as, in fact, are all nuclei whose mass numbers A and charges Z are both even.
It is not so unfortunate as it might seem that the principal isotope of carbon can give no nuclear magnetic resonance signal, since if 12C had a sizable nuclear moment the proton NMR spectra of most organic compounds would be much more complicated than they actually are. Furthermore, 13C has a magnetic moment so that when there is vital necessity for observing a carbon resonance signal, 13C can usually be used, either at its prevailing low natural concentration or with the aid of 13C-enriched material.
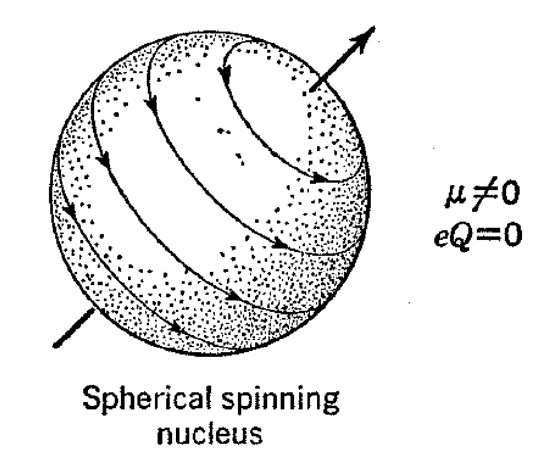
A number of nuclei of particular importance to organic chemistry may be assigned nuclear spin values of 1/2. This means that they act as though they were spherical bodies possessing uniform charge distributions but spinning like tops. A spinning nucleus has circulating charge, and this generates a magnetic field so that a nuclear magnetic moment results. The spherical charge distribution ascribed to nuclei with spin of 1/2 means that a probing charge approaching them experiences the same electrostatic field regardless of the direction of approach and, therefore, as with the spherical nonspinning nuclei, the electric quadrupole moment is zero. Nuclei with a spin of 1/2 include 1H, 13C, 15N, 19F, and 31P, and, in general, such nuclei are particularly favorable for nuclear resonance experiments.
A very large number of magnetic nuclei act as though they are spinning bodies with nonspherical charge distributions and are assigned spin values of unity or larger integral multiples of 1/Z. Often such nuclei are taken to approximate ellipsoids spinning about the principal axis. A charged, elongated (prolate) ellipsoid with the charge uniformly distributed over its surface will present an anisotropic electrostatic field to an approaching unit charge so that the electrostatic work will be different in bringing up a unit charge to a given distance if the charge approaches along the spin axis or at some angle to it. By convention, the electric quadrupole moment of a nucleus ascribed the shape of a prolate ellipsoid is assigned a value greater than zero. Important examples are 2H and 14N.
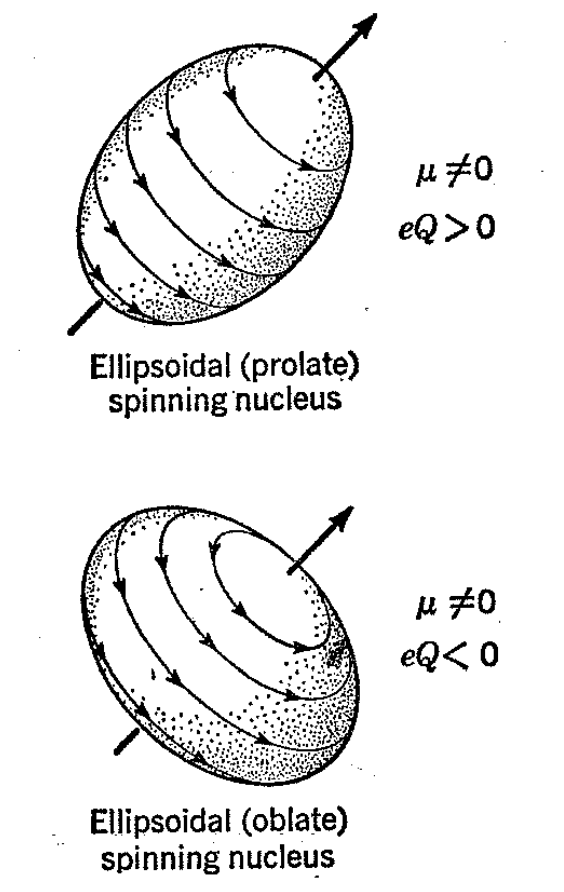
Nuclei which behave like charged, flattened (oblate) ellipsoids also present an anisotropic electric field to a probing charge and by convention are assigned negative electric-quadrupole-moment values. Nuclei of this type include 17O, 33S, 35Cl, etc. In the ensuing discussion, we shall confine our attention largely to nuclei with a spin of 1/2, since, as will be seen, complications are often introduced when the electric quadrupole moment is different from zero. These complications are in themselves capable of providing useful chemical information but are not helpful to an understanding of the operation of a nuclear resonance spectrometer.


