9.5: Hydration- Oxymercuration-Demercuration
- Page ID
- 45203
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objective
- apply the principles of regioselectivity and stereoselectivity to the addition reactions of alkenes
- predict the products, specify the reagents, and discern most efficient reaction for hydration of alkenes (acid catalyzed hydration; or oxymercuration/demercuration; or hydroboration/oxidation)
Introduction
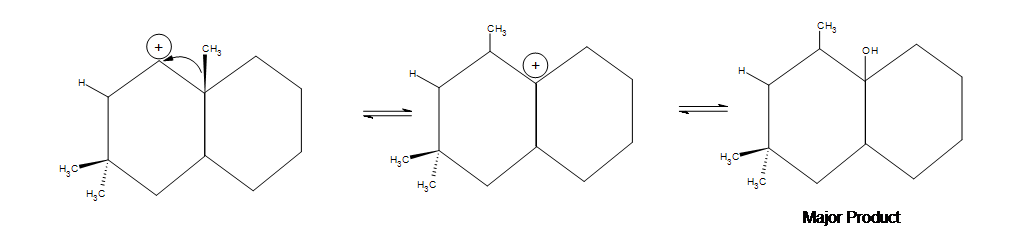
Acid-catalyzed hydration of alkenes is limited by carbocation stability. Carbocation rearrangement can occur to form a more stable ion as shown in the example below.
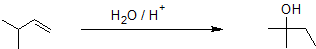
Alkene hydration using the oxymercuration-demercuration reaction pathway reliably produces the Markovnikov product without carbocation rearrangment as shown in the example below.
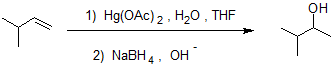
Oxymercuration-Demercuration is a two step pathway used to produce alcohols.
Oxymercuration-Demercuration Mechanism
This mechanism is similar to the previous electrophilic addition reactions. The major difference is that a mercurium ion bridge stabilizes the carbocation intermediate so that it cannot rearrange. Metals are electropositive. Mercury carries a partial positive charge in the acetate complex and is the electrophile. During the first step of this mechanism, the pi electrons form a bond to mercury while the lone pair on the mercury simultaneously bonds to the other vinyl carbon creating a mercurium ion bridge. The mercurium ion forms in conjunction with the loss of an acetate ion. The mercurium ion stabilizes the carbocation so that it does not rearrange. In the second step of this mechanism, a water molecule reacts with the most substituted carbon to open the mercurium ion bridge. The third step of this mechanism is a proton transfer to a solvent water molecule to neutralize the addition product. The fourth step of the reaction pathway is the reduction of the organomercury intermediate with sodium borohydride under basic conditions. The mechanism of the fourth step is beyond the scope of first year organic chemistry.
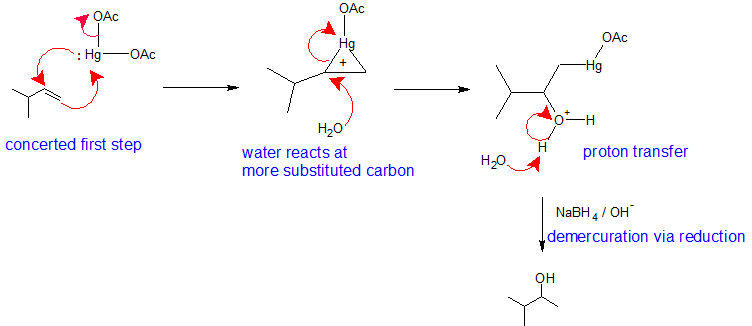
Notice that overall, the oxymercuration - demercuration mechanism follows Markovnikov's Regioselectivity with the OH group attached to the most substituted carbon and the H attached to the least substituted carbon. The reaction is useful, because strong acids are not required and carbocation rearrangements are avoided because no discreet carbocation intermediate forms.
Exercise
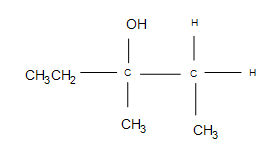
1. Show how to prepare 3-methyl2-pentanol from 3-methyl-1-pentene.
Note: Questions 2-5 have not shown the water present in the sulfuric acid solution and have indicated a second neutralization step. Some authors simply write H+/H2O as a single step.
2. Draw the bond-line structure for the product.
.bmp?revision=1&size=bestfit&width=326&height=100)
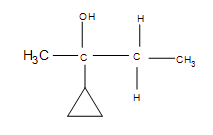
3. Draw the bond-line structure for the product. How does the cyclopropane group affect the reaction?
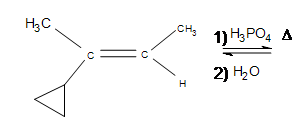
4. Draw the bond-line structure for the product. (Hint: What is different about this problem?)
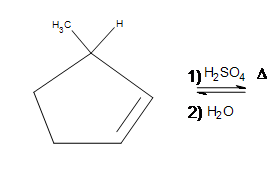
5. Draw the bond-line structure for the product(s). Indicate any shifts as well as the major product:

6. In each case, predict the product(s) of these reactants of oxymercuration.
7. Propose the alkene that was the reactant for each of these products of oxymercuration.
- Answer
-
1.
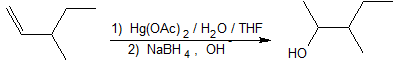
2. This reaction is electrophilic hydration.
3. The answer is additional side products, but the major product formed is still the same (the product shown). Depending on the temperatures used, the cyclopropane may open up into a straight chain, which makes it unlikely that the major product will form (after the reaction, it is unlikely that the 3º carbon will remain as such).
4. A hydride shift actually occurs from the top of the 1-methylcyclopentane to where the carbocation had formed.
.bmp?revision=1&size=bestfit&width=290&height=201)
5. In the first picture shown below, an alkyl shift occurs but a hydride shift (which occurs faster) is possible. Why doesn't a hydride shift occur? The answer is because the alkyl shift leads to a more stable product. There is a noticeable amount of side product that forms where the two methyl groups are, but the major product shown below is still the most significant due to the hyperconjugation that occurs by being in between the two cyclohexanes.
6.
7.
References
- Vollhardt, K. Peter C. Organic chemistry structure and function. New York: W.H. Freeman, 2007.
- Smith, Michael B., and Jerry March. March's Advanced Organic Chemistry Reactions, Mechanisms, and Structure (March's Advanced Organic Chemistry). New York: Wiley-Interscience, 2007 2007.
- Roderic P. Quirk , Robert E. Lea, Reductive demercuration of hex-5-enyl-1-mercuric bromide by metal hydrides. Rearrangement, isotope effects, and mechanism, J. Am. Chem. Soc., 1976, 98 (19), pp 5973–5978.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- Lance Peery (UCD), Duyen Dao-Tran (UCD)
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
Jim Clark (Chemguide.co.uk)
Prof. Steven Farmer (Sonoma State University)