24.1: Names and Structures of Carbohydrates
- Page ID
- 32567
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Objectives
After completing this section, you should be able to
- classify a specific carbohydrate as being a monosaccharide, disaccharide, trisaccharide, etc., given the structure of the carbohydrate or sufficient information about its structure.
- classify a monosaccharide according to the number of carbon atoms present and whether it contains an aldehyde or ketone group.
Make certain that you can define, and use in context, the key terms below.
- aldose
- disaccharide
- ketose
- monosaccharide (simple sugar)
- polysaccharide
What Are Carbohydrates?
The most abundant biomolecules on earth are carbohydrates. From a chemical viewpoint, carbohydrates are primarily a combination of carbon and water, and many of them have the empirical formula (CH2O)n, where n is the number of repeated units. This view represents these molecules simply as “hydrated” carbon atom chains in which water molecules attach to each carbon atom, leading to the term “carbohydrates.” Although all carbohydrates contain carbon, hydrogen, and oxygen, there are some that also contain nitrogen, phosphorus, and/or sulfur. Carbohydrates have myriad different functions. They are abundant in terrestrial ecosystems, many forms of which we use as food sources. These molecules are also vital parts of macromolecular structures that store and transmit genetic information (i.e., DNA and RNA). They are the basis of biological polymers that impart strength to various structural components of organisms (e.g., cellulose and chitin), and they are the primary source of energy storage in the form of starch and glycogen.
Monosaccharides
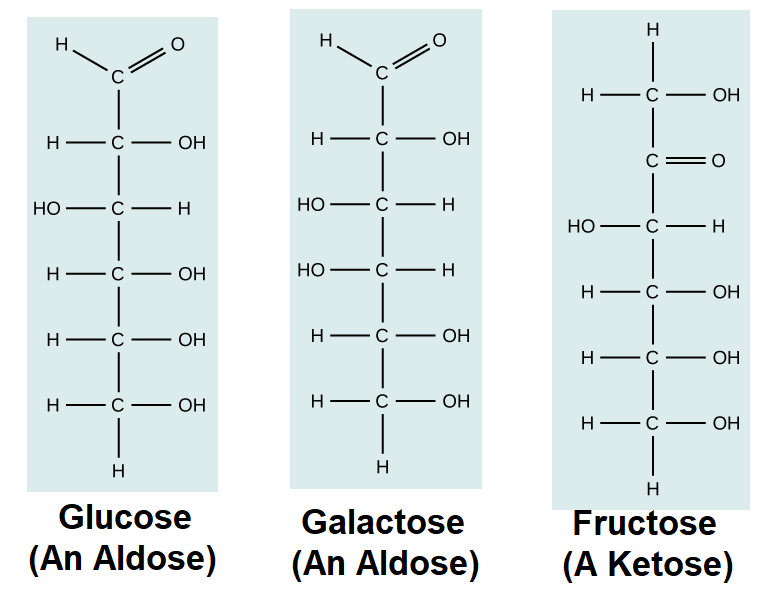
In biochemistry, carbohydrates are often called saccharides, from the Greek sakcharon, meaning sugar, although not all the saccharides are sweet. The simplest carbohydrates are called monosaccharides, or simple sugars. They are the building blocks (monomers) for the synthesis of polymers or complex carbohydrates, as will be discussed further in this section. Monosaccharides are classified based on the number of carbons in the molecule. General categories are identified using a prefix that indicates the number of carbons and the suffix –ose, which indicates a saccharide; for example, triose (three carbons), tetrose (four carbons), pentose (five carbons), and hexose (six carbons). The hexose D-glucose is the most abundant monosaccharide in nature. Other very common and abundant hexose monosaccharides are galactose, used to make the disaccharide milk sugar lactose, and the fruit sugar fructose.
A second comparison can be made when looking at glucose, galactose, and fructose. All three are hexoses; however, there is a major structural difference between glucose and galactose versus fructose: the carbon that contains the carbonyl (C=O). In glucose and galactose, the carbonyl group is on the C1 carbon, forming an aldehyde group. In fructose, the carbonyl group is on the C2 carbon, forming a ketone group. The former sugars are called aldoses based on the aldehyde group that is formed; the latter is designated as a ketose based on the ketone group. Again, this difference gives fructose different chemical and structural properties from those of the related aldoses, glucose, and galactose, even though fructose, glucose, and galactose all have the same chemical composition: C6H12O6.
Complex Carbohydrates
The simple sugars form the foundation of more complex carbohydrates. The cyclic forms of two sugars can be linked together by means of a condensation reaction to form a disaccharide. Multiple sugars can be linked to form polysaccharides.
Disaccharides
Two monosaccharide molecules may chemically bond to form a disaccharide. The name given to the covalent bond between the two monosaccharides is a glycosidic bond. Glycosidic bonds form between hydroxyl groups of the two saccharide molecules, an example of the dehydration synthesis described later in this chapter.

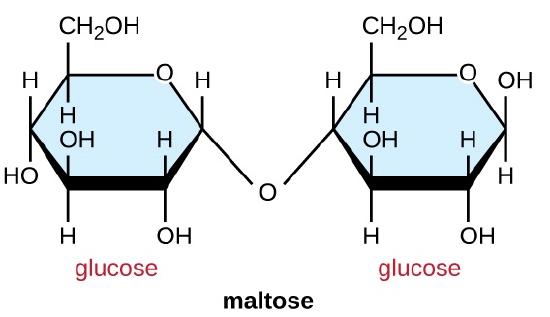
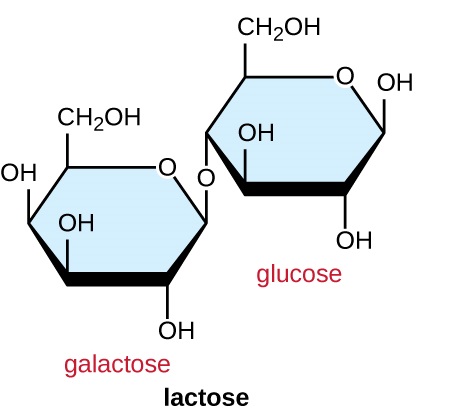
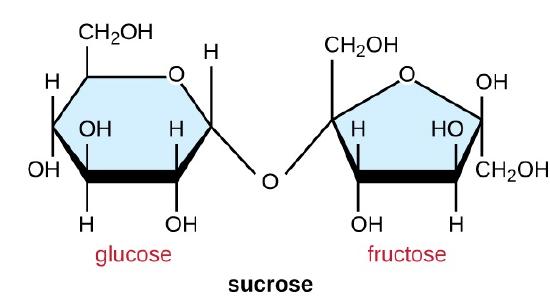
Common disaccharides are the grain sugar maltose, made of two glucose molecules; the milk sugar lactose, made of one galactose and one glucose molecule; and the table sugar sucrose, made of one glucose and one fructose molecule.
 nosaccharide—OH+HO—monosaccharide⟶monosaccharide—O—monosaccharidedisaccharide
nosaccharide—OH+HO—monosaccharide⟶monosaccharide—O—monosaccharidedisaccharidePolysaccharides
Polysaccharides, also called glycans, are large polymers composed of hundreds of monosaccharide monomers. Unlike mono- and disaccharides, polysaccharides are not sweet and, in general, they are not soluble in water. Like disaccharides, the monomeric units of polysaccharides are linked together by glycosidic bonds.
Polysaccharides are very diverse in their structure. Three of the most biologically important polysaccharides—starch, glycogen, and cellulose—are all composed of repetitive glucose units, although they differ in their structure. Cellulose consists of a linear chain of glucose molecules and is a common structural component of cell walls in plants and other organisms. Glycogen and starch are branched polymers; glycogen is the primary energy-storage molecule in animals and bacteria, whereas plants primarily store energy in starch. The orientation of the glycosidic linkages in these three polymers is different as well and, as a consequence, linear and branched macromolecules have different properties.
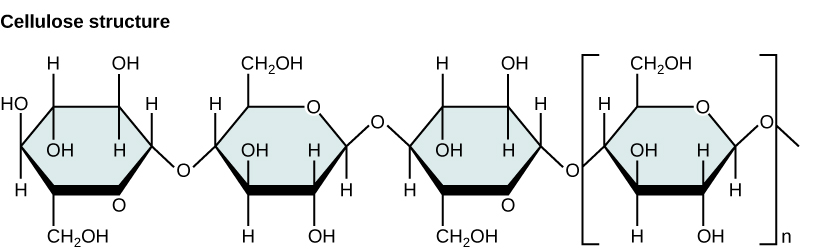
Summary
|
||||||
|
||||||
|
Exercise
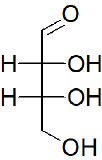
Classify each of the following sugars.
a)
b)
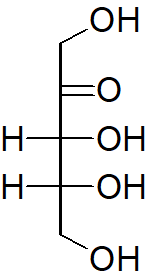
c)
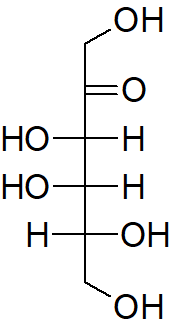
d)
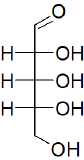
- Answer
-
a) Aldoterose
b) Ketopentose
c) Ketohexose
d) Aldopentose
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Objectives
After completing this section, you should be able to
- draw the Fischer projection of a monosaccharide, given its wedge‑and‑broken‑line structure or a molecular model.
- draw the wedge‑and‑broken‑line structure of a monosaccharide, given its Fischer projection or a molecular model.
- construct a molecular model of a monosaccharide, given its Fischer projection or wedge‑and‑broken‑line structure.
Make certain that you can define, and use in context, the key term below.
- Fischer projection
When studying this section, use your molecular model set to assist you in visualizing the structures of the compounds that are discussed. It is important that you be able to determine whether two apparently different Fischer projections represent two different structures or one single structure. Often the simplest way to check is to construct a molecular model corresponding to each projection formula, and then compare the two models.
The problem of drawing three-dimensional configurations on a two-dimensional surface, such as a piece of paper, has been a long-standing concern of chemists. The wedge and hatched line notations we have been using are effective, but can be troublesome when applied to compounds having many chiral centers. As part of his Nobel Prize-winning research on carbohydrates, the great German chemist Emil Fischer, devised a simple notation that is still widely used. In a Fischer projection drawing, the four bonds to a chiral carbon make a cross with the carbon atom at the intersection of the horizontal and vertical lines. The two horizontal bonds are directed toward the viewer (forward of the stereogenic carbon). The two vertical bonds are directed behind the central carbon (away from the viewer). Since this is not the usual way in which we have viewed such structures, the following diagram shows how a stereogenic carbon positioned in the common two-bonds-in-a-plane orientation ( x–C–y define the reference plane ) is rotated into the Fischer projection orientation (the far right formula). When writing Fischer projection formulas it is important to remember these conventions. Since the vertical bonds extend away from the viewer and the horizontal bonds toward the viewer, a Fischer structure may only be turned by 180º within the plane, thus maintaining this relationship. The structure must not be flipped over or rotated by 90º.
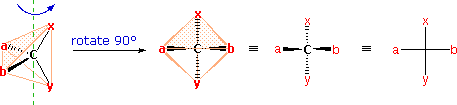
In the above diagram, if x = CO2H, y = CH3, a = H & b = OH, the resulting formula describes (R)-(–)-lactic acid. The mirror-image formula, where x = CO2H, y = CH3, a = OH & b = H, would, of course, represent (S)-(+)-lactic acid.
The Fischer Projection consists of both horizontal and vertical lines, where the horizontal lines represent the atoms that are pointed toward the viewer while the vertical line represents atoms that are pointed away from the viewer. The point of intersection between the horizontal and vertical lines represents the central carbon.
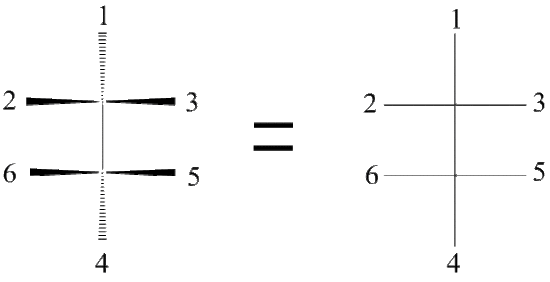
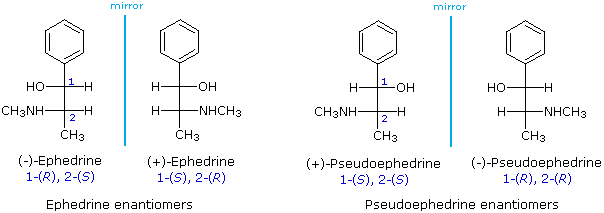
Using the Fischer projection notation, the stereoisomers of 2-methylamino-1-phenylpropanol are drawn in the following manner. Note that it is customary to set the longest carbon chain as the vertical bond assembly.
The usefulness of this notation to Fischer, in his carbohydrate studies, is evident in the following diagram. There are eight stereoisomers of 2,3,4,5-tetrahydroxypentanal, a group of compounds referred to as the aldopentoses. Since there are three chiral centers in this constitution, we should expect a maximum of 23 stereoisomers. These eight stereoisomers consist of four sets of enantiomers. If the configuration at C-4 is kept constant (R in the examples shown here), the four stereoisomers that result will be diastereomers. Fischer formulas for these isomers, which Fischer designated as the "D"-family, are shown in the diagram. Each of these compounds has an enantiomer, which is a member of the "L"-family so, as expected, there are eight stereoisomers in all. Determining whether a chiral carbon is R or S may seem difficult when using Fischer projections, but it is actually quite simple. If the lowest priority group (often a hydrogen) is on a vertical bond, the configuration is given directly from the relative positions of the three higher-ranked substituents. If the lowest priority group is on a horizontal bond, the positions of the remaining groups give the wrong answer (you are in looking at the configuration from the wrong side), so you simply reverse it.
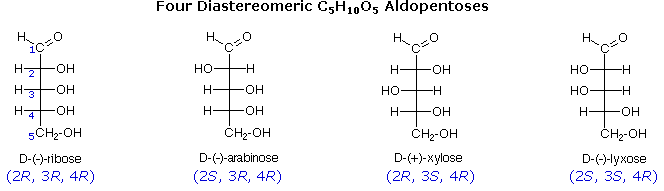
The aldopentose structures drawn above are all diastereomers. A more selective term, epimer, is used to designate diastereomers that differ in configuration at only one chiral center. Thus, ribose and arabinose are epimers at C-2, and arabinose and lyxose are epimers at C-3. However, arabinose and xylose are not epimers, since their configurations differ at both C-2 and C-3.
How to make Fischer Projections
To make a Fischer Projection, it is easier to show through examples than through words. Lets start with the first example, turning a 3D structure of ethane into a 2D Fischer Projection.
Start by mentally converting a 3D structure into a Dashed-Wedged Line Structure. Remember, the atoms that are pointed toward the viewer would be designated with a wedged lines and the ones pointed away from the viewer are designated with dashed lines.
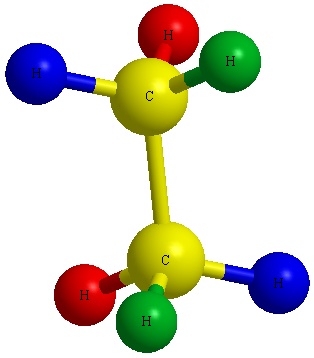
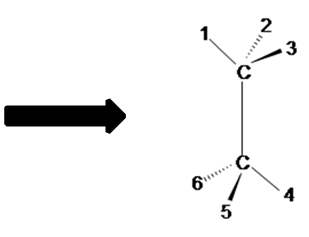
Figure A Figure B
Notice the red balls (atoms) in Figure A above are pointed away from the screen. These atoms will be designated with dashed lines like those in Figure B by number 2 and 6. The green balls (atoms) are pointed toward the screen. These atoms will be designated with wedged lines like those in Figure B by number 3 and 5. The blue atoms are in the plane of the screen so they are designated with straight lines.
Now that we have our Dashed- Wedged Line Structure, we can convert it to a Fischer Projection. However, before we can convert this Dashed-Wedged Line Structure into a Fischer Projection, we must first convert it to a “flat” Dashed-Wedged Line Structure. Then from there we can draw our Fischer Projection. Lets start with a more simpler example. Instead of using the ethane shown in Figure A and B, we will start with a methane. The reason being is that it allows us to only focus on one central carbon, which make things a little bit easier.
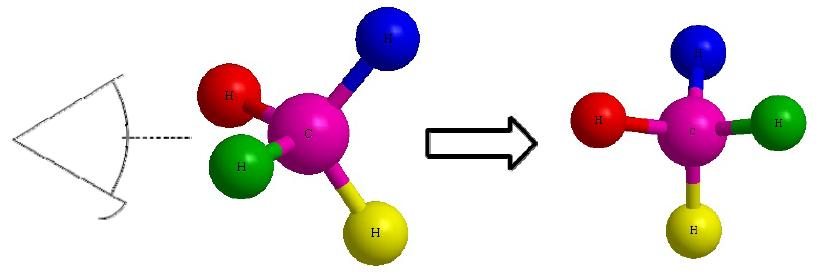
Figure C Figure D
Lets start with this 3D image and work our way to a dashed-wedged image. Start by imagining yourself looking directly at the central carbon from the left side as shown in Figure C. It should look something like Figure D. Now take this Figure D and flatten it out on the surface of the paper and you should get an image of a cross.
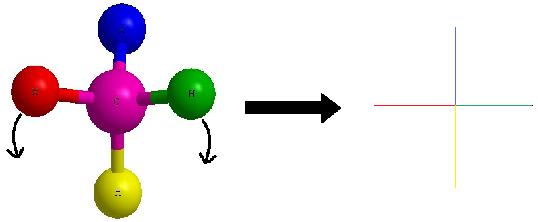
As a reminder, the horizontal line represents atoms that are coming out of the paper and the vertical line represents atoms that are going into the paper. The cross image to the right of the arrow is a Fischer projection.
Exercises
Exercise 25.2.1
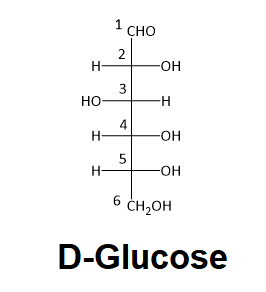
Determine if carbon #2 in D-glucose is R or S.
- Answer
-
When deciding whether a stereocenter in a Fischer projection is R or S, realize that the hydrogen, in a horizontal bond. Therefore, the orientation of the three remaining substituents is reversed to create the correct answer or a counterclockwise circle means R, and a clockwise circle means S. For carbon #2 in D-Glucose substituent 1, 2, and 3 form a counterclockwise circle so the carbon is R.
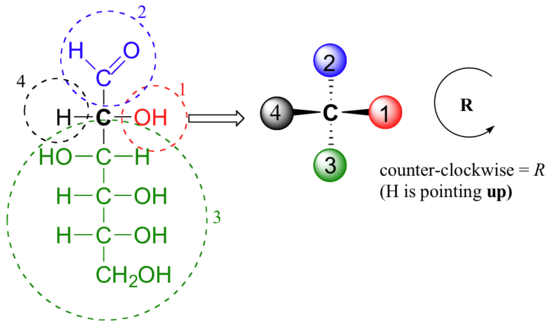
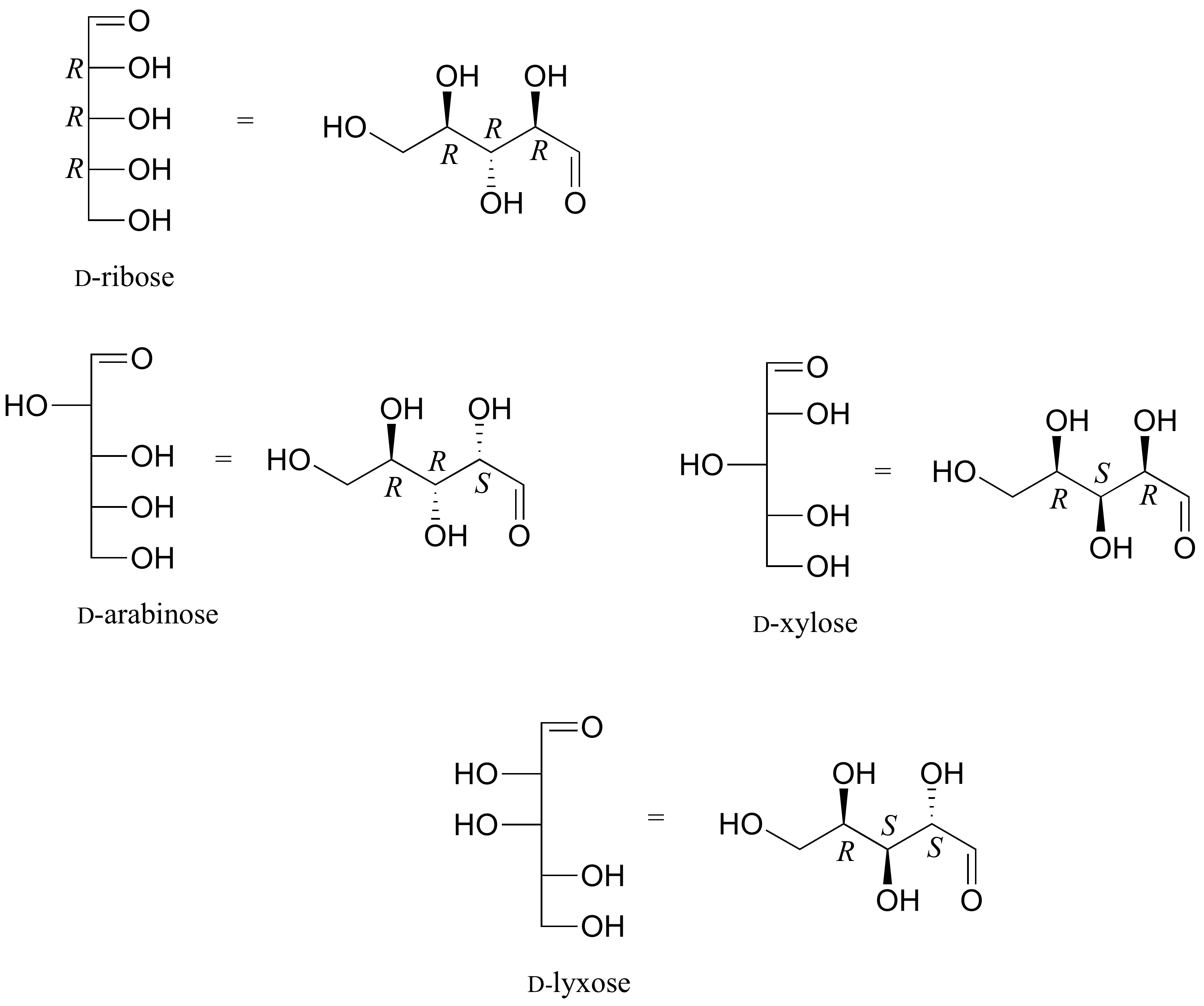
Draw 'zigzag' structures (using the solid/dash wedge convention to show stereochemistry) for the four sugars in the figure below. Label all stereocenters R or S.
- Answer
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Objectives
After completing this section, you should be able to
- identify a specific enantioner of a monosaccharide as being D or L, given its Fischer projection.
- identify the limitations of the D, L system of nomenclature for carbohydrates.
- assign an R or S configuration to each of the chiral carbon atoms present in a monosaccharide, given its Fischer projection.
- draw the Fischer projection formula for a monosaccharide, given its systematic name, complete with the configuration of each chiral carbon atom.
- construct a molecular model of a monosaccharide, given its systematic name, complete with the configuration of each chiral carbon atom.
Make certain that you can define, and use in context, the key terms below.
- D sugar
- L sugar
If you find that you have forgotten the meanings of terms such as dextrorotatory and polarimeter, refer back to Section 5.3 in which the fundamentals of optical activity were introduced.
How would you set about the task of deciding whether each chiral carbon has an R or an S configuration? True, you could use molecular models, but suppose that a model set had not been available—what would you have done then?
One approach is to focus on the carbon atom of interest and sketch a three-dimensional representation of the configuration around that atom, remembering the convention used in Fischer projections: vertical lines represent bonds going into the page, and horizontal lines represent bonds coming out of the page. Thus, the configuration around carbon atom 2 in structure a can be represented as follows:
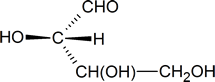
In your mind, you should be able to imagine how this molecule would look if it was rotated so that the bonds that are shown as coming out of the page are now in the plane of the page. [One possible way of doing this is to try and imagine how the molecule would look if it was viewed from a point at the bottom of the page.] What you should see in your mind is a representation similar to the one drawn below.
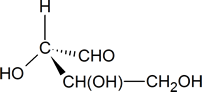
To determine whether the configuration about the central carbon atom is R or S, we must rotate the molecule so that the group with the lowest priority (H), is directed away from the viewer. This effect can be achieved by keeping the hydroxyl group in its present position and moving each of the other three groups one position clockwise.
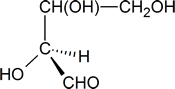
The Cahn-Ingold-Prelog order of priority for the three remaining groups is OH > CHO > CH(OH)CH2OH; thus, we see that we could trace out a counterclockwise path going from the highest-priority group to the second- and third-highest, and we conclude that the central carbon atom has an S configuration.
The Configuration of Glucose
The four chiral centers in glucose indicate there may be as many as sixteen (24) stereoisomers having this constitution. These would exist as eight diastereomeric pairs of enantiomers, and the initial challenge was to determine which of the eight corresponded to glucose. This challenge was accepted and met in 1891 by the German chemist Emil Fischer. His successful negotiation of the stereochemical maze presented by the aldohexoses was a logical tour de force, and it is fitting that he received the 1902 Nobel Prize for chemistry for this accomplishment. One of the first tasks faced by Fischer was to devise a method of representing the configuration of each chiral center in an unambiguous manner. To this end, he invented a simple technique for drawing chains of chiral centers, that we now call the Fischer projection formula. Click on this link for a review.
At the time Fischer undertook the glucose project it was not possible to establish the absolute configuration of an enantiomer. Consequently, Fischer made an arbitrary choice for (+)-glucose and established a network of related aldose configurations that he called the D-family. The mirror images of these configurations were then designated the L-family of aldoses. To illustrate using present day knowledge, Fischer projection formulas and names for the D-aldose family (three to six-carbon atoms) are shown below, with the asymmetric carbon atoms (chiral centers) colored red. The last chiral center in an aldose chain (farthest from the aldehyde group) was chosen by Fischer as the D / L designator site. If the hydroxyl group in the projection formula pointed to the right, it was defined as a member of the D-family. A left directed hydroxyl group (the mirror image) then represented the L-family. Fischer's initial assignment of the D-configuration had a 50:50 chance of being right, but all his subsequent conclusions concerning the relative configurations of various aldoses were soundly based. In 1951 x-ray fluorescence studies of (+)-tartaric acid, carried out in the Netherlands by Johannes Martin Bijvoet, proved that Fischer's choice was correct.
It is important to recognize that the sign of a compound's specific rotation (an experimental number) does not correlate with its configuration (D or L). It is a simple matter to measure an optical rotation with a polarimeter. Determining an absolute configuration usually requires chemical interconversion with known compounds by stereospecific reaction paths.
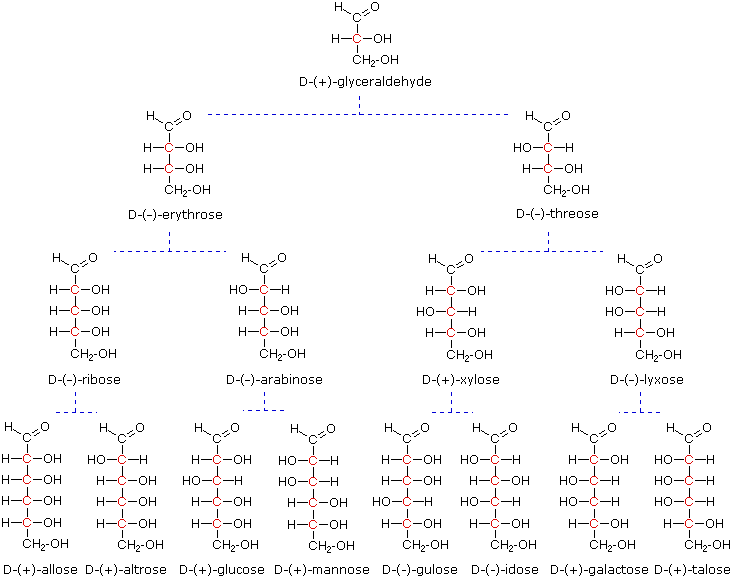
Exercise
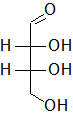
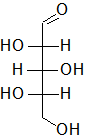
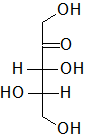
In the following Fischer projections, assign R and S for each chiral center and determine whether each sugar is a D or L sugar.
a)
b)
c)
- Answer
-
a) From top to bottom, 2R, 3R, and it is a D sugar.
b) From top to bottom, 2S, 3R, 4S, and it is an L sugar.
c) From to to bottom, 3R, 4S, and it is an L sugar.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Objectives
After completing this section, you should be able to
- draw the structures of all possible aldotetroses, aldopentoses, and aldohexoses, without necessarily being able to assign names to the individual compounds.
- draw the Fischer projection of D‑glyceraldehyde, D‑ribose and D‑glucose from memory.
The four chiral centers in glucose indicate there may be as many as sixteen (24) stereoisomers having this constitution. These would exist as eight diastereomeric pairs of enantiomers, and the initial challenge was to determine which of the eight corresponded to glucose. This challenge was accepted and met in 1891 by the German chemist Emil Fischer. His successful negotiation of the stereochemical maze presented by the aldohexoses was a logical tour de force, and it is fitting that he received the 1902 Nobel Prize for chemistry for this accomplishment. One of the first tasks faced by Fischer was to devise a method of representing the configuration of each chiral center in an unambiguous manner. To this end, he invented a simple technique for drawing chains of chiral centers, that we now call the Fischer projection formula.
At the time Fischer undertook the glucose project it was not possible to establish the absolute configuration of an enantiomer. Consequently, Fischer made an arbitrary choice for (+)-glucose and established a network of related aldose configurations that he called the D-family. The mirror images of these configurations were then designated the L-family of aldoses. To illustrate using present day knowledge, Fischer projection formulas and names for the D-aldose family (three to six-carbon atoms) are shown below, with the asymmetric carbon atoms (chiral centers) colored red.
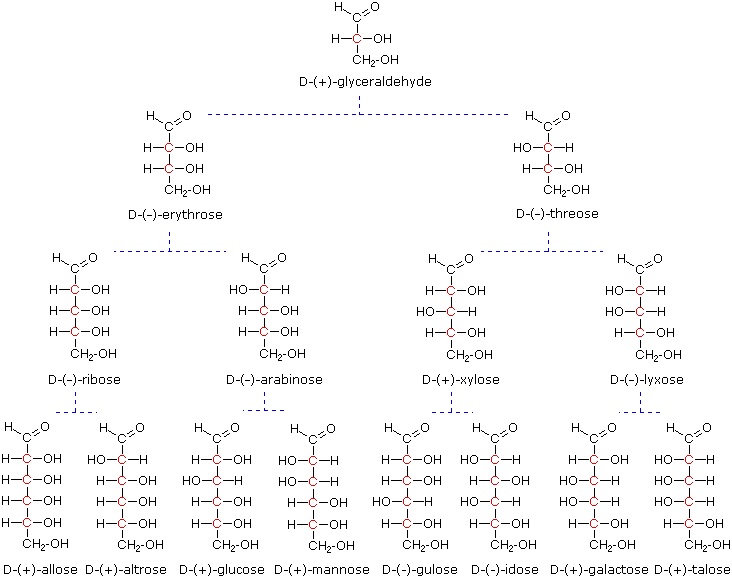
The last chiral center in an aldose chain (farthest from the aldehyde group) was chosen by Fischer as the D / L designator site. If the hydroxyl group in the projection formula pointed to the right, it was defined as a member of the D-family. A left directed hydroxyl group (the mirror image) then represented the L-family. Fischer's initial assignment of the D-configuration had a 50:50 chance of being right, but all his subsequent conclusions concerning the relative configurations of various aldoses were soundly based. In 1951 x-ray fluorescence studies of (+)-tartaric acid, carried out in the Netherlands by Johannes Martin Bijvoet (pronounced "buy foot"), proved that Fischer's choice was correct.
It is important to recognize that the sign of a compound's specific rotation (an experimental number) does not correlate with its configuration (D or L). It is a simple matter to measure an optical rotation with a polarimeter. Determining an absolute configuration usually requires chemical interconversion with known compounds by stereospecific reaction paths.
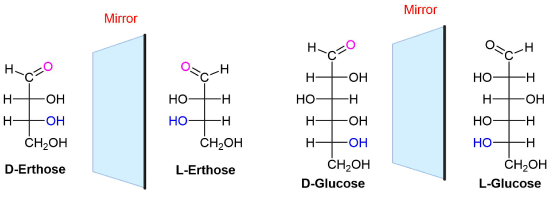
Draw the Fisher projection of L-erythrose and L-Glucose
Solution
Use the Fischer projection provided above and reverse all of the chiral centers to provide the L-sugar. Note that in both cases the D sugars have the OH going to the right on the chiral center furthest away from the carbonyl. The L-sugars have the OH going to the left.
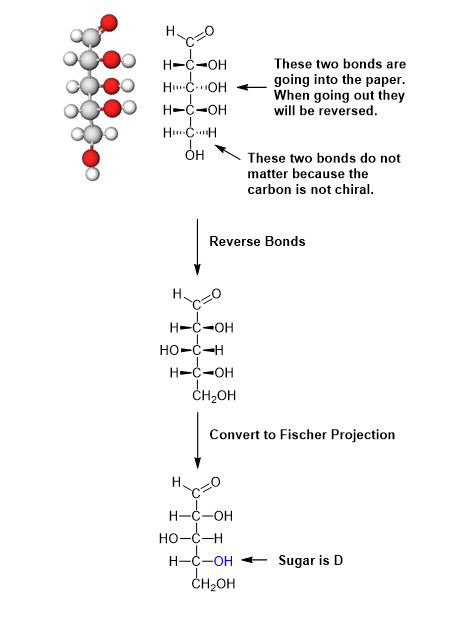
Please draw the Fischer projection fo the following aldopentose and determine if the sugars is D or L.
Solution
First, rotate the model so that the carbonyl is at the top. This is requirement of a Fischer projection. Next rotate the model so that the H and OH of the chiral carbon just below the carbonyl are facing towards you. In this orientation, a dash/wedge model will have every other set of bonds going into the plane of the page. This is not the correct orientation of a Fischer project so they must be modified. The H and OH bonds need to be coming out of the plane of the page in a Fisher projects. When converting bonds from going into the page to going out of the page the orientation of the H and OH are reversed. Remember that the last -CH2OH of a sugar is achiral so the orientation does not need to be shown. Once the bonds are oriented correctly the wedge bonds can be converted to those of a Fischer projection.
Exercises
For the following model of a sugar, please draw its Fischer projection and name it.
- Answer
-
D-Mannose
How many heptose stereoisomers would there expected to be? How many would be D-Sugars?
- Answer
-
There would be 25 = 32 heptose stereoisomers. Half of these would be D-sugars or 16.
Draw the Fischer projection of the following sugars.
- L-Ribose
- L-Galactose
- L-Talose
- Answer
-
a)
b)
c)
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry

