20.1: Relative Reactivities, Structures, and Spectra of Carboxylic Acid Derivatives
- Page ID
- 32534
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Objectives
After completing this section, you should be able to
- compare the reactions of carboxylic acid derivatives with nucleophiles to the reactions of aldehydes and ketones with nucleophiles.
- arrange a given list of carboxylic acid derivatives in order of increasing or decreasing reactivity towards nucleophiles.
- explain the difference in reactivity towards nucleophiles of two or more given carboxylic acid derivatives.
- explain why esters and amides are commonly found in nature, but acid halides and acid anhydrides are not.
The general nucleophilic acyl substitution reaction, and its mechanism, were discussed earlier in “III. General Reactions of Carbonyl Compounds.” Review if necessary.
The reading describes the relative reactivities of “biologically relevant acyl groups.” Both acid anhydrides and acid halides readily react with water and cannot exist for any length of time in living organisms. The following scheme illustrates the relative reactivities of most carboxylic acid derivatives that you will encounter.
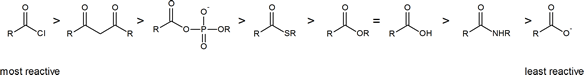
Carboxylic acid derivatives and acyl groups
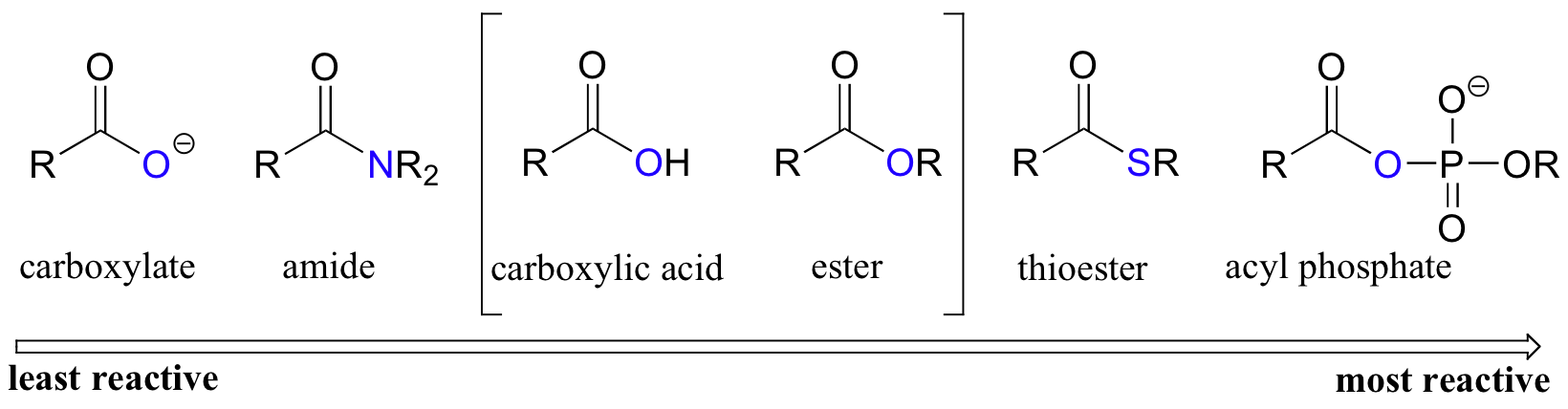
The functional groups that undergo nucleophilic acyl substitutions are called carboxylic acid derivatives: these include carboxylic acids themselves, carboxylates (deprotonated carboxylic acids), amides, esters, thioesters, and acyl phosphates. Two more examples of carboxylic acid derivatives which are less biologically relevant but important in laboratory synthesis are carboxylic acid anyhydrides and acid chlorides.
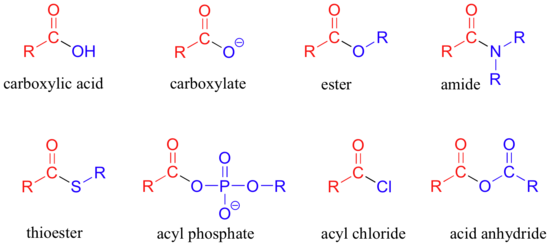
The carboxylic acid derivatives can be distinguished from aldehydes and ketones by the presence of a group containing an electronegative heteroatom - usually oxygen, nitrogen, or sulfur – bonded directly to the carbonyl carbon. You can think of a carboxylic acid derivative as having two sides. One side is the carbonyl group and the attached alkyl group: this is called an acyl group (in the specific case where R is a methyl group, the term acetyl group is used). One the other side is the heteroatom-containing group: in this text, we will sometimes refer to this component as the ‘acyl X' group (this, however, is not a standard term in organic chemistry).
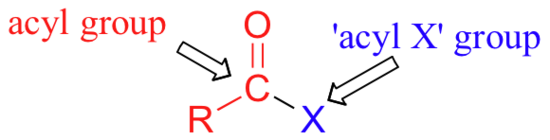
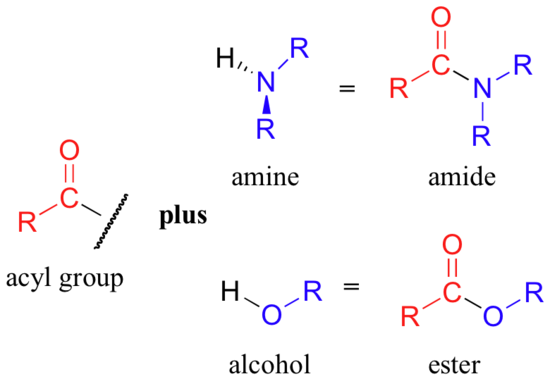
Notice that the acyl X groups are simply deprotonated forms of other functional groups: in an amide, for example, the acyl X group is an amine, while in an ester the acyl X group is an alcohol.
What is the ‘Y’ group in:
- an acid anhydride?
- a carboxylic acid?
Answers
a.
b.
The nucleophilic acyl substitution reaction
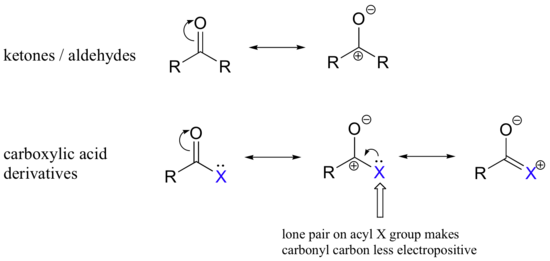
The fact that the atom adjacent to the carbonyl carbon in carboxylic acid derivatives is an electronegative heteroatom – rather than a carbon like in ketones or a hydrogen like in aldehydes - is critical to understanding the reactivity of these functional groups. Just like in aldehydes and ketones, carboxylic acid derivatives are attacked from one side of their trigonal planar carbonyl carbon by a nucleophile, converting this carbon to tetrahedral (sp3) geometry. In carboxylic acid derivatives, the acyl X group is a potential leaving group. What this means is that the tetrahedral product formed from attack of the nucleophile on the carbonyl carbon is not the product: it is a reactive intermediate. The tetrahedral intermediate rapidly collapses: the carbon-oxygen double bond re-forms, and the acyl X group is expelled.

Notice that in the product, the nucleophile becomes the new acyl X group. This is why this reaction type is called a nucleophilic acyl substitution: one acyl X group is substituted for another. For example, in the reaction below, one alcohol X group (3-methyl-1-butanol) is replaced by another alcohol X group (methanol), as one ester is converted to another.
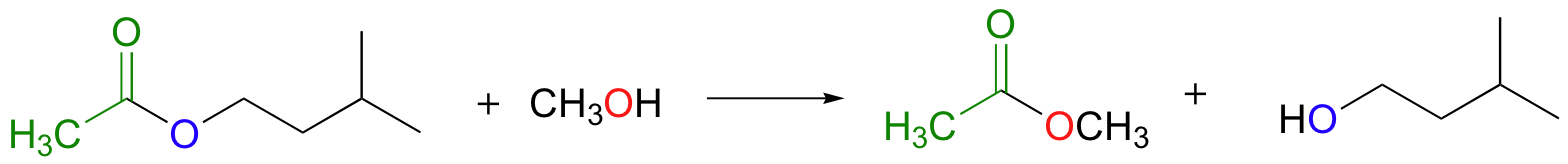
Another way of looking at this reaction is to picture the acyl group being transferred from one acyl X group to another: in the example above, the acetyl group is being transferred from 3-methyl-1-butanol to methanol. For this reason, nucleophilic acyl substitutions are also commonly referred to as acyl transfer reactions.
When the incoming nucleophile in an acyl substitution is a water molecule, the reaction is also referred to as an acyl hydrolysis. For example, the following reaction can be described as the hydrolysis of an ester (to form a carboxylic acid and an alcohol).
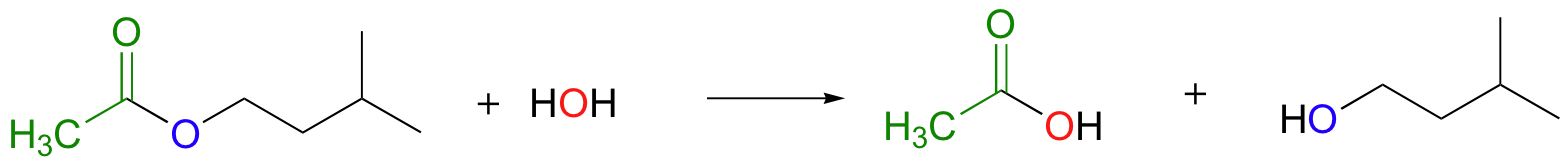
We could also describe this reaction as the transfer of an acyl group from an alcohol to a water molecule.
In a similar vein, the hydrolysis of an amide to form a carboxylic acid could be described as the transfer of an acyl group from ammonia (NH3) to water.
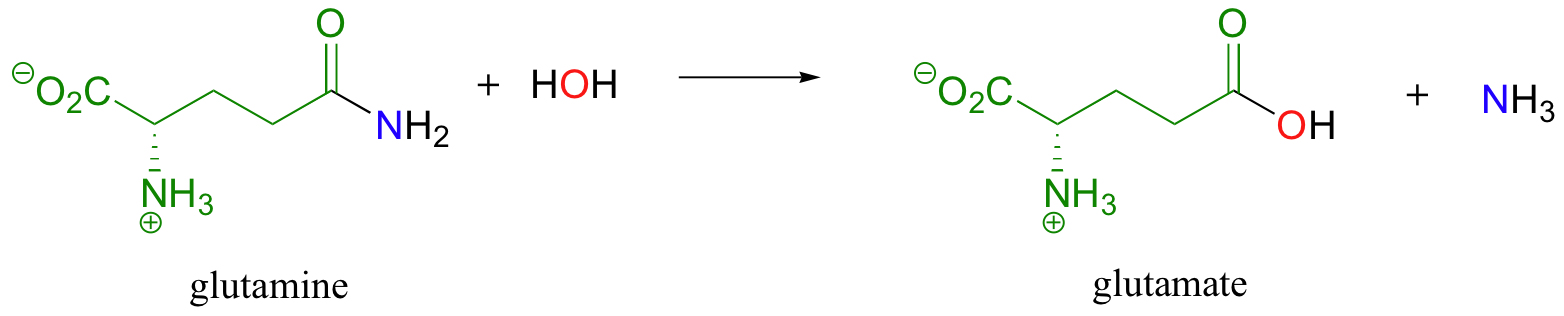
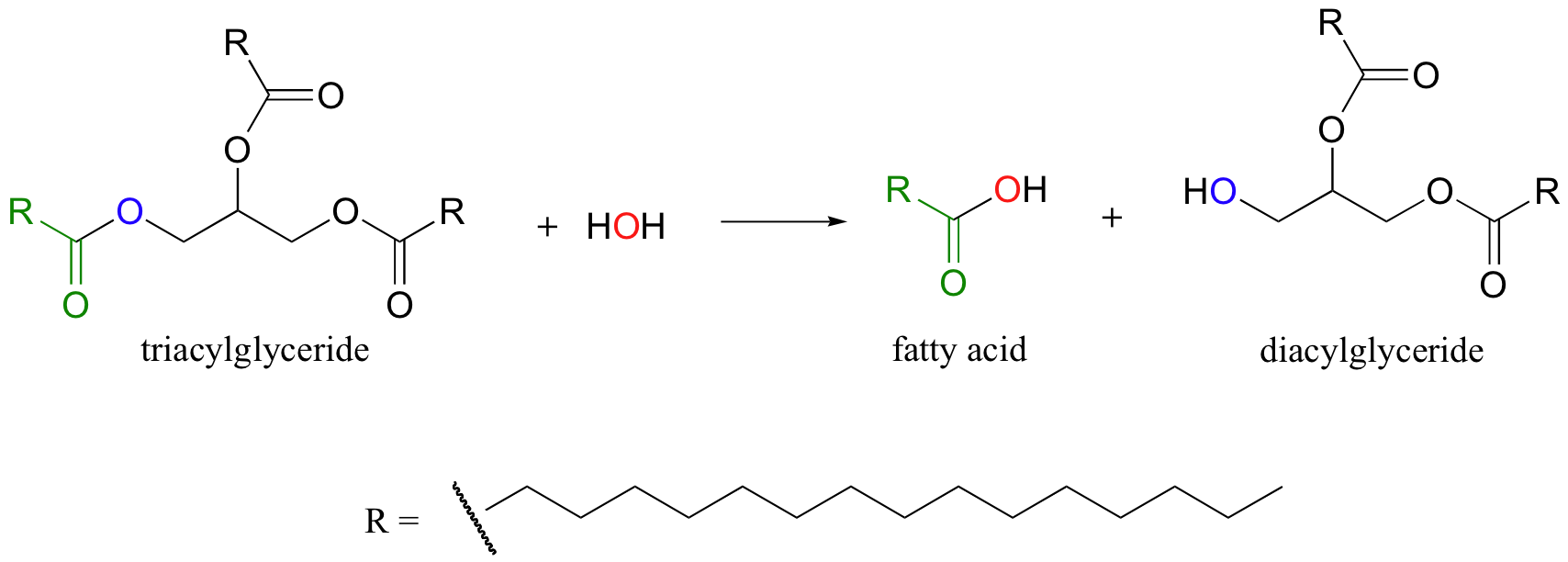
As we will see in later sections of this chapter the hydrolysis of esters and amides are particularly important reaction types in biochemical pathways. When your body digests the fat in a hamburger, for example, enzymes in your pancreas called lipases first catalyze ester hydrolysis reactions to free the fatty acids (we'll look more closely at this reaction in section 12.4D).
The relative reactivity of carboxylic acid derivatives
The relative reactivity of the carboxylic acid derivatives is an important concept to understand before entering into a detailed examination of nucleophilic acyl substitutions. As a general rule, the carbonyl carbon in an acyl group is less electrophilic than that in an aldehyde or ketone. This is because in carboxylic acid derivatives, the partial positive charge on the carbon is stabilized somewhat by resonance effects from the adjacent heteroatom.
Among the carboxylic acid derivatives, carboxylate groups are the least reactive towards nucleophilic acyl substitution, followed by amides, then esters and (protonated) carboxylic acids, thioesters, and finally acyl phosphates, which are the most reactive among the biologically relevant acyl groups.
The different reactivities of the functional groups can be understood by evaluating the basicity of the leaving group in each case - remember from section 8.5 that weaker bases are better leaving groups! A thioester is more reactive than an ester, for example, because a thiolate (RS-) is a weaker base than an alkoxide (RO-). In general, if the incoming nucleophile is a weaker base than the ‘acyl X’ group that is already there, the first nucleophilic step will simply reverse itself and we’ll get the starting materials back:
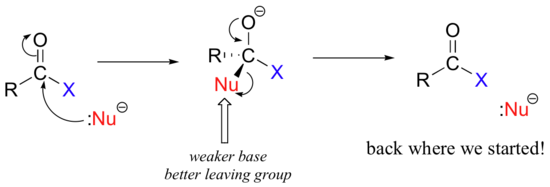
This is why it is not possible to directly convert an ester, for example, into a thioester by an acyl substitution reaction – this would be an uphill reaction.
Here’s another way to think about the relative reactivites of the different carboxylic acid derivatives: consider the relative electrophilicity, or degree of partial positive charge, on the carbonyl carbon in each species. This depends on how much electron density the neighboring heteroatom on the acyl X group is able to donate: greater electron donation by the heteroatom implies lower partial positive charge on the carbonyl carbon, which in turn implies lower electrophilicity.
The negatively charged oxygen on the carboxylate group has lots of electron density to donate, thus the carbonyl carbon is not very electrophilic. In amides, the nitrogen atom is a powerful electron donating group by resonance - recall that the carbon-nitrogen bond in peptides has substantial double-bond character - thus amides are relatively unreactive. Amides do undergo acyl substitution reactions in biochemical pathways, but these reactions are inherently slow and the enzymes catalyzing them have evolved efficient strategies to lower the activation energy barrier.
Carboxylic acids and esters are in the middle range of reactivity, while thioesters are somewhat more reactive. The most reactive of the carboxylic acid derivatives frequently found in biomolecules are the acyl phosphates. These are most often present in two forms: the simple acyl monophosphate, and the acyl-adenosine monophosphate.
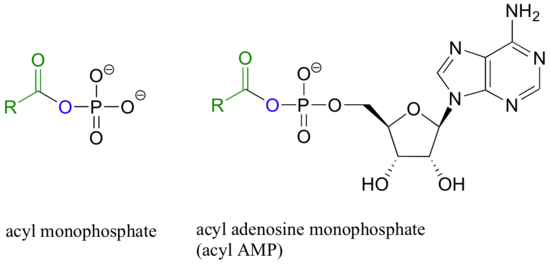
Both are highly reactive to acyl substitution reactions, and are often referred to as ‘activated acyl groups’ or ‘activated carboxylic acids’. The high reactivity of acyl phosphates is due mainly to the ability to form complexes with magnesium ions.
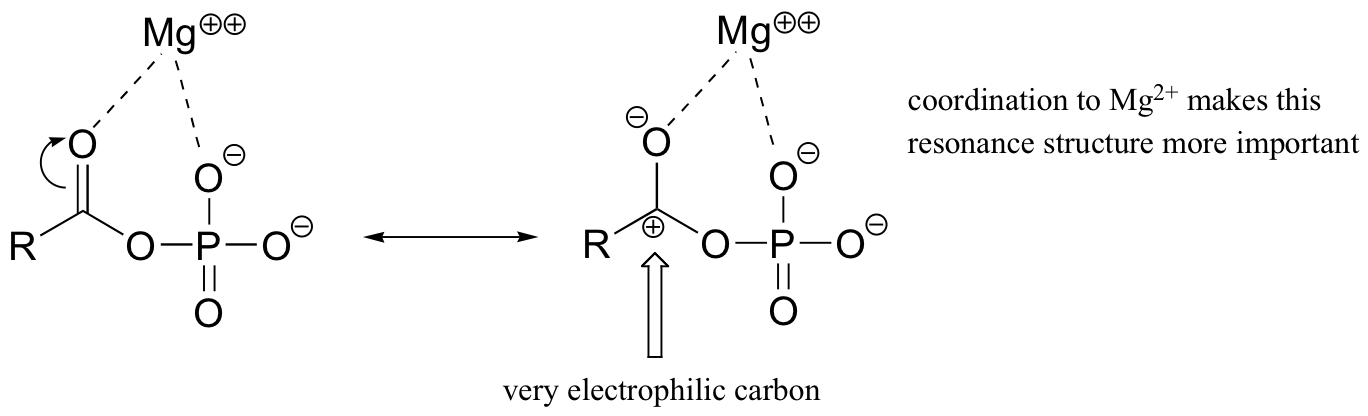
The magnesium ion acts as a Lewis acid to accept electron density from the oxygen end of the acyl carbonyl bond, thus greatly increasing the degree of partial positive charge - and thus the electrophilicity - of the carbonyl carbon. The magnesium ion also balances negative charge on the phosphate, making it an excellent leaving group.
In our examination of acyl substitution reactions, we will start with the formation and reactions of the highly reactive acyl phosphates. We will then discuss how thioesters play a key role in the acyl substitution reactions of lipid metabolism. Finally, we will take a look at some important acyl substitution reactions involving esters, as well as the formation and cleavage of the amide linkages in the peptide bonds of proteins.
Draw the expected products of the following reactions:
a)
b)
Solution
a) The nucleophile is neutral.
b) The nucleophile is negatively charged.
Exercises
Which of the following compounds would be expected to be the most reactive towards nucleophilic acyl substitution? Briefly explain your answer.
a)
b)
- Answer
-
a) Compound A would expected to be the more reactive. For two compounds with the same carboxylic acid derivative group the one with the least steric crowding on the acyl side is typically the most reactive.
b) Compound B would be expected to be the more reactive. In general acid anhydrides are more reactive than ester.
Draw the expected products of the following reactions:
a)
b)
c)
- Answer
-
a)
b)
c)
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)

