15.5: Polycyclic Aromatic Hydrocarbons
- Page ID
- 32476
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to draw the resonance contributors for polycyclic aromatic compounds, such as naphthalene, anthracene, etc.
Make certain that you can define, and use in context, the key term below.
- polycyclic aromatic compounds
As their name indicates, polycyclic aromatic hydrocarbons are aromatic hydrocarbons which contain more than one benzenoid (i.e., benzene-like) ring. This section deals only with those compounds in which the benzenoid rings are fused together; in other words, compounds in which at least one carbon-carbon bond is common to two aromatic rings. Another type of polycyclic aromatic hydrocarbon contains two or more benzenoid rings joined by a carbon-carbon single bond. The simplest compound of this type is biphenyl, the compound from which PCBs (polychlorinated biphenyls) are derived.
Polycyclic Aromatic Compounds
Hückel's 4n +2 rule for aromaticity does not only apply to mono-cyclic compounds. Benzene rings may be joined together (fused) to give larger polycyclic aromatic compounds. A few examples are drawn below, together with the approved numbering scheme for substituted derivatives. The peripheral carbon atoms (numbered in all but the last three examples) are all bonded to hydrogen atoms. Unlike benzene, all the C-C bond lengths in these fused ring aromatics are not the same, and there is some localization of the pi-electrons. The six benzene rings in coronene are fused in a planar ring; whereas the six rings in hexahelicene are not joined in a larger ring, but assume a helical turn, due to the crowding together of the terminal ring atoms (in the structure below, note that the top right and center right rings are not attached to one another). This helical configuration renders the hexahelicene molecule chiral, and it has been resolved into stable enantiomers.
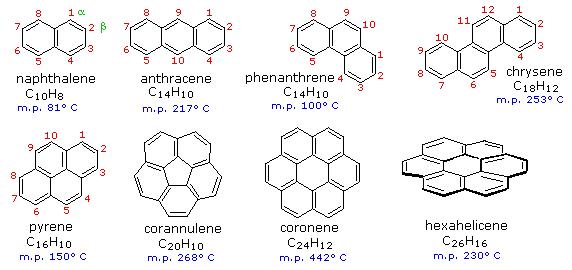
Figure 2: Examples of Polycyclic Aromatic Hydrocarbons (PAHs).
Probably the most well know polycyclic aromatic compound which only contains carbon is naphthalene (C10H8). Naphthalene shares many of the same characteristic as benzene. Naphthalene is cyclic and known to be flat. Each carbon in naphthalene is sp2 hybridized so it is completely conjugated. The true structure of naphthalene is a combination of three resonance hybrids.
Naphthalene
Heat of hydrogenation experiments with naphthalene shows an unusual "aromatic" stabilization energy. Also, naphthalene prefers to react with electrophiles to give substitution products rather than the typical double bond addition products.
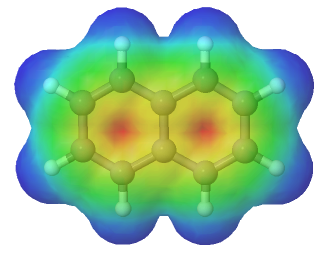
Every carbon in naphthalene is sp2 hybridized so there is conjugated p orbital overlap over the entire ring system. The electrostatic potential map shows that pi electrons in naphthalene are evenly distributed making each carbon equivalent.
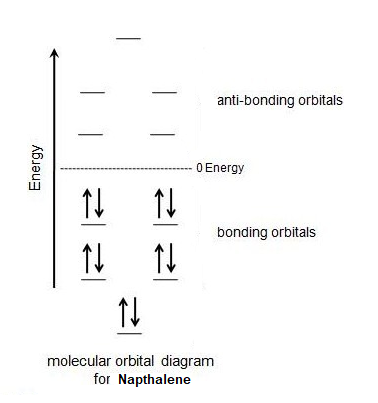
Lastly, naphthalene has 10 pi electrons which fulfills Hückel's rule. The importance of the 4n + 2 rule can be seen when considering the molecular orbital diagram of naphthalene. Naphthalene has 10 p orbitals which is 4 more than benzene. In the molecular orbital diagram of naphthalene, the 4 additional p orbitals become 2 bonding orbitals and two anti-bonding orbitals. The two additional bonding orbitals require 4 additional pi electrons to be filled so naphthalene needs 10 pi electrons in total to fill all of the bonding molecular orbitals.
Polycyclic Aromatic Heterocycles
There is a wide variety of polycyclic aromatic heterocycles, many of which contain nitrogen, oxygen, or sulfur. Some of the biologically important structures, are the nitrogen containing polycyclic aromatic heterocycles indole, quinoline, isoquinoline, and purine which are all polycyclic aromatic heterocycles commonly found in nature. Notice that these compound all have 10 pi electrons.

Indole, quinoline, and isoquinoline all contain a hetrocyclic ring fused to benzene. Purine is made up to two heterocyclic rings, imidazole and pyrimidine, fused together. Quinoline is found in the antimalarial drug quinine. Indole is found in the neurotransmitter serotonin. The purine ring structure is found in adenine and guanine, two important parts of DNA and in the stimulant caffeine.
Exercises
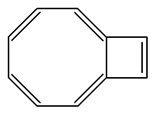
1) The following molecule is an isomer of naphthalene. Is it aromatic? Draw a resonance structure for it.
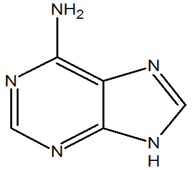
2) The following molecule is adenine. It has a purine core. Of the nitrogen in the core, how many electrons are donated into the pi system?
- Answer
-
1) It has 10 pi electrons which follows the 4n+2 rule making it aromatic.
2) There is only one nitrogen of the core that contributes a set of lone pair electrons as 2 pi electrons (in red). All of the other nitrogens are sp2 hybrized and contribute 1 pi electron each. In total, the core is aromatic with 10 electrons in the pi-system.
This is an isomer of naphthalene. Is it aromatic? Draw a resonance structure for it.
- Answer
-
Yes, it is aromatic. 4n+2 pi-electrons.
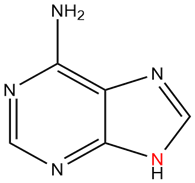
The following molecule is adenine. It has a purine core. Of the nitrogen in the core, how many electrons are donated into the pi system?
- Answer
-
There is only one nitrogen of the core that contributes to the pi-system (in red). With this one lone pair the core is aromatic with 10 electrons in the pi-system.
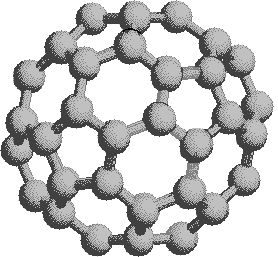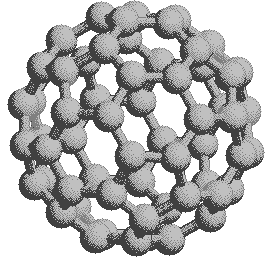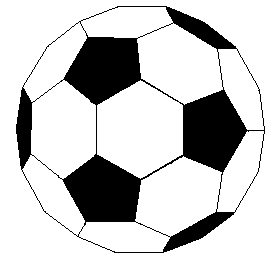
If we extend the structure of corannulene by adding similar cycles of five benzene rings, the curvature of the resulting molecule should increase, and eventually close into a sphere of carbon atoms. The archetypical compound of this kind (C60) has been named buckminsterfullerene because of its resemblance to the geodesic structures created by Buckminster Fuller. It is a member of a family of similar carbon structures that are called fullerenes. These materials represent a third class of carbon allotropes. Alternating views of the C60 fullerene structure are shown on the right, together with a soccer ball-like representation of the 12 five and 20 six-membered rings composing its surface. Precise measurement by Atomic Force Microscopy (AFM) has shown that the C-C bond lengths of the six-membered rings are not all equal, and depend on whether the ring is fused to a five or six-membered beighbor. By clicking on this graphic, a model of C60 will be displayed.
Although C60 is composed of fused benzene rings its chemical reactivity resembles that of the cycloalkenes more than benzene. Indeed, exposure to light and oxygen slowly degrade fullerenes to cage opened products. Most of the reactions thus far reported for C60 involve addition to, rather than substitution of, the core structure. These reactions include hydrogenation, bromination and hydroxylation. Strain introduced by the curvature of the surface may be responsible for the enhanced reactivity of C60.
. Larger fullerenes, such as C70, C76, C82 & C84have ellipsoidal or distorted spherical structures, and fullerene-like assemblies up to C240 have been detected. A fascinating aspect of these structures is that the space within the carbon cage may hold atoms, ions or small molecules. Such species are called endohedral fullerenes. The cavity of C60 is relatively small, but encapsulated helium, lithium and atomic nitrogen compounds have been observed. Larger fullerenes are found to encapsulate lanthanide metal atoms.
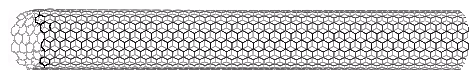
Interest in the fullerenes has led to the discovery of a related group of carbon structures referred to as nanotubes. As shown in the following illustration, nanotubes may be viewed as rolled up segments of graphite. The chief structural components are six-membered rings, but changes in tube diameter, branching into side tubes and the capping of tube ends is accomplished by fusion with five and seven-membered rings. Many interesting applications of these unusual structures have been proposed.