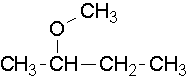9.5: Names and Physical Properties of Ethers
- Page ID
- 32402
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Ethers are compounds having two alkyl or aryl groups bonded to an oxygen atom, as in the formula R1–O–R2. The ether functional group does not have a characteristic IUPAC nomenclature suffix, so it is necessary to designate it as a substituent. To do so the common alkoxy substituents are given names derived from their alkyl component (below):
| Alkyl Group | Name | Alkoxy Group | Name | |
|---|---|---|---|---|
| CH3– | Methyl | CH3O– | Methoxy | |
| CH3CH2– | Ethyl | CH3CH2O– | Ethoxy | |
| (CH3)2CH– | Isopropyl | (CH3)2CHO– | Isopropoxy | |
| (CH3)3C– | tert-Butyl | (CH3)3CO– | tert-Butoxy | |
| C6H5– | Phenyl | C6H5O– | Phenoxy |
Ethers can be named by naming each of the two carbon groups as a separate word followed by a space and the word ether. The -OR group can also be named as a substituent using the group name, alkox
CH3-CH2-O-CH3 is called ethyl methyl ether or methoxyethane.
The smaller, shorter alkyl group becomes the alkoxy substituent. The larger, longer alkyl group side becomes the alkane base name. Each alkyl group on each side of the oxygen is numbered separately. The numbering priority is given to the carbon closest to the oxgen. The alkoxy side (shorter side) has an "-oxy" ending with its corresponding alkyl group. For example, CH3CH2CH2CH2CH2-O-CH2CH2CH3 is 1-propoxypentane. If there is cis or trans stereochemistry, the same rule still applies.
- \(CH_3CH_2OCH_2CH_3\), diethyl ether (sometimes referred to as just ether)
- \(CH_3OCH_2CH_2OCH_3\), ethylene glycol dimethyl ether (glyme).
Common names
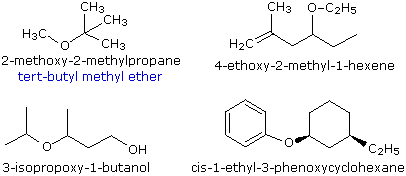
Simple ethers are given common names in which the alkyl groups bonded to the oxygen are named in alphabetical order followed by the word "ether". The top left example shows the common name in blue under the IUPAC name. Many simple ethers are symmetrical, in that the two alkyl substituents are the same. These are named as "dialkyl ethers".
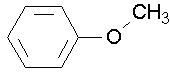
- anisole (try naming anisole by the other two conventions. J )
- oxirane
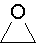
1,2-epoxyethane, ethylene oxide, dimethylene oxide, oxacyclopropane,
- furan (this compound is aromatic)
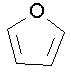
tetrahydrofuran

oxacyclopentane, 1,4-epoxybutane, tetramethylene oxide,
- dioxane

1,4-dioxacyclohexane
Heterocycles
In cyclic ethers (heterocycles), one or more carbons are replaced with oxygen. Often, it's called heteroatoms, when carbon is replaced by an oxygen or any atom other than carbon or hydrogen. In this case, the stem is called the oxacycloalkane, where the prefix "oxa-" is an indicator of the replacement of the carbon by an oxygen in the ring. These compounds are numbered starting at the oxygen and continues around the ring. For example,

If a substituent is an alcohol, the alcohol has higher priority. However, if a substituent is a halide, ether has higher priority. If there is both an alcohol group and a halide, alcohol has higher priority. The numbering begins with the end that is closest to the higher priority substituent. There are ethers that are contain multiple ether groups that are called cyclic polyethers or crown ethers. These are also named using the IUPAC system.
Sulfides
Sulfur analogs of ethers (R–S–R') are called sulfides, e.g., (CH3)3C–S–CH3 is tert-butyl methyl sulfide. Sulfides are chemically more reactive than ethers, reflecting the greater nucleophilicity of sulfur relative to oxygen.
References
- Schore, Neil E. and Vollhardt, K. Peter C. Organic Chemistry: Structure and Function. New York: Bleyer, Brennan, 2007.
- Winter, Arthur. Organic Chemistry for Dummies. Hoboken, New Jersey: Wiley, 2005.
- Pellegrini, Frank. Cliffs QuickReview Organic Chemistry II. Foster City, CA: Wiley, 2000
Problems
Name the following ethers:
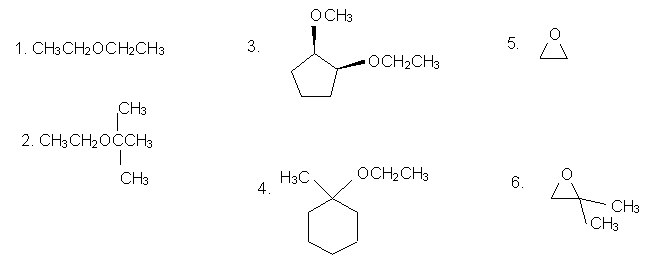
(Answers to problems above: 1. diethyl ether; 2. 2-ethoxy-2-methyl-1-propane; 3. cis-1-ethoxy-2-methoxycyclopentane; 4. 1-ethoxy-1-methylcyclohexane; 5. oxacyclopropane; 6. 2,2-Dimethyloxacyclopropane)
(CC-BY; Dan Johnson via Twitter)
- Answer
-
A one-eyed one-horned flying propyl people ether
Contributors
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
After completing this section, you should be able to
- write the normally accepted name for a crown ether, given its structure.
- draw the structure of a crown ether, given its normally accepted name.
- describe, briefly, the uses of crown ethers.
Make certain that you can define, and use in context, the key term below.
- crown ether
A “crown ether ” is a cyclic ether containing several (i.e., 4, 5, 6 or more) oxygen atoms. As we have indicated in the objectives above, a detailed knowledge of these compounds is not required in this course.
Crown Ethers
Crown ethers are cyclic polyethers with four or more oxygen atoms each separated by two or three carbon atoms. Crown ethers have the general formula of (OCH2CH2)n or (OCH2CH2CH2)n and are named using both the total number of atoms in the ring and the number of oxygen atoms. Thus 18-crown-6 is an 18-membered ring with six oxygen atoms. All crown ethers have a cavity in the center this is lined with oxygen atoms and can accommodate a alkali metal ion, such as K+. The cation is stabilized by interacting with lone pairs of electrons on the surrounding oxygen atoms. The negative character of center cavity of 18-crown-6 can be seen by looking at its electrostatic potential map shown below. The presence of high electron density is shown by a red color.
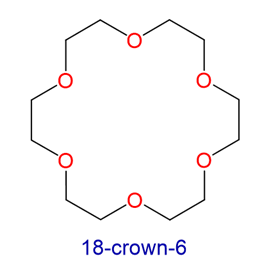
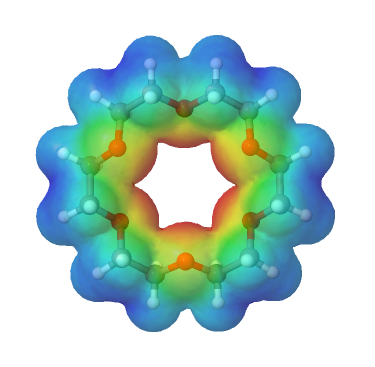
Crown ethers are useful for dissolving ionic substances in organic solvents, such as KMnO4 dissolving in toluene, by sequestering the cations inside a hydrophilic cavity, whereas the outer shell, consisting of C–H bonds, is hydrophobic.
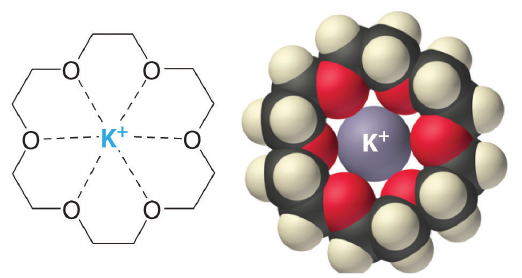
The availability of crown ethers with cavities of different sizes allows specific cations to be solvated with a high degree of selectivity. Crown ethers prefer to bind alkali metal cations with sizes that match that of their binding cavity. For instance, as shown in Table \(\PageIndex{1}\), 14-crown-4 preferentially binds to Li+, 15-crown-5 preferentially binds to Na+, 18-crown-6 preferentially binds to K+, and 21-crown-7 preferentially binds to Cs+.
| Crown Ether | Cavity Diameter (A˚) | Preferred Cation | Cation Diameter (A˚) |
|---|---|---|---|
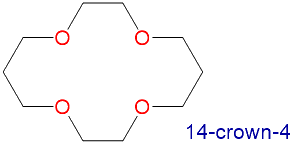 |
1.2-1.5 | Li+ | 1.36 |
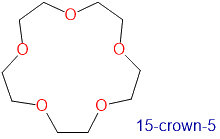 |
1.7-2.2 | Na+ | 1.94 |
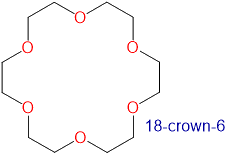 |
2.6-3.2 | K+ | 2.66 |
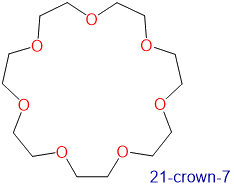 |
3.4-4.3 | Cs+ | 3.34 |
Cryptands
Cryptands (from the Greek kryptós, meaning “hidden”) are variations of crown ethers comprised of two nitrogens connected by three polyether strands. A common nomenclature is used where the numbers preceding the word cryptan indicate the number of oxygen atoms in each strand of the molecule.
Like crown ethers, cryptands are compounds that contains a central cavity that can completely surround a cation with lone pair electrons from oxygen and nitrogen atoms. Also, cryptands can be used to prepare solutions of ionic compounds in solvents that are otherwise too nonpolar to dissolve them. Similar to crown ethers, cryptands prefer to bind with alkali metal cations whose diameter matches the size of their binding cavity.
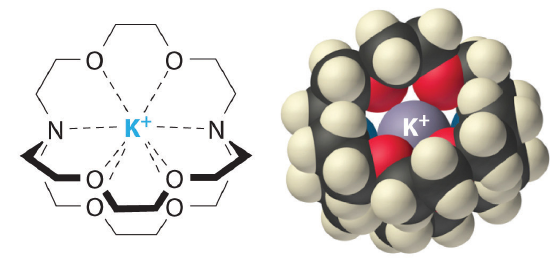
Cryptand ligands also preferentially bind alkali metal ions with sizes that match the size of their binding cavities. The structure and approximate cavity sizes of several cryptands are shown above. Use the information in Table \(\PageIndex{1}\) to predict which alkali metal ion each cryptand will preferentially bind.
- Answer
-
The cryptands might be expected to selectively bind the largest ion which fits within the cavity. Predicted selectivity of crown ethers for alkali metal cations based on the hypothesis that they will selectively bind the largest ion which fits their binding cavity are listed below.
Cryptand Cavity Diameter of (Å) Preferred Cation Cation Diameter (Å) 2.1.1-cryptand 1.60 Li+ 1.36 2.2.1-cryptand 2.20 Na+ 1.96 2.2.2-cryptand 2.80 K+ 2.66 3.2.2-cryptand 3.60 Cs+ 3.34