4.13: Oxidation of Alkanes
- Page ID
- 30351
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Oxidation and Reduction of Alkanes
You are undoubtedly already familiar with the general idea of oxidation and reduction: you learned in general chemistry that when a compound or atom is oxidized it loses electrons, and when it is reduced it gains electrons. You also know that oxidation and reduction reactions occur in pairs: if one species is oxidized, another must be reduced at the same time - thus the term 'redox reaction'.
Most of the redox reactions you have seen previously in general chemistry probably involved the flow of electrons from one metal to another, such as the reaction between copper ion in solution and metallic zinc:
Cu+2(aq) + Zn(s) → Cu(s) + Zn+2(aq)
In organic chemistry, redox reactions look a little different. Electrons in an organic redox reaction often are transferred in the form of a hydride ion - a proton and two electrons. Because they occur in conjunction with the transfer of a proton, these are commonly referred to as hydrogenation and dehydrogenation reactions: a hydride plus a proton adds up to a hydrogen (H2) molecule. Be careful - do not confuse the terms hydrogenation and dehydrogenation with hydration and dehydration - the latter refer to the gain and loss of a water molecule (and are not redox reactions), while the former refer to the gain and loss of a hydrogen molecule.
When a carbon atom in an organic compound loses a bond to hydrogen and gains a new bond to a heteroatom (or to another carbon), we say the compound has been dehydrogenated, or oxidized. A very common biochemical example is the oxidation of an alcohol to a ketone or aldehyde:
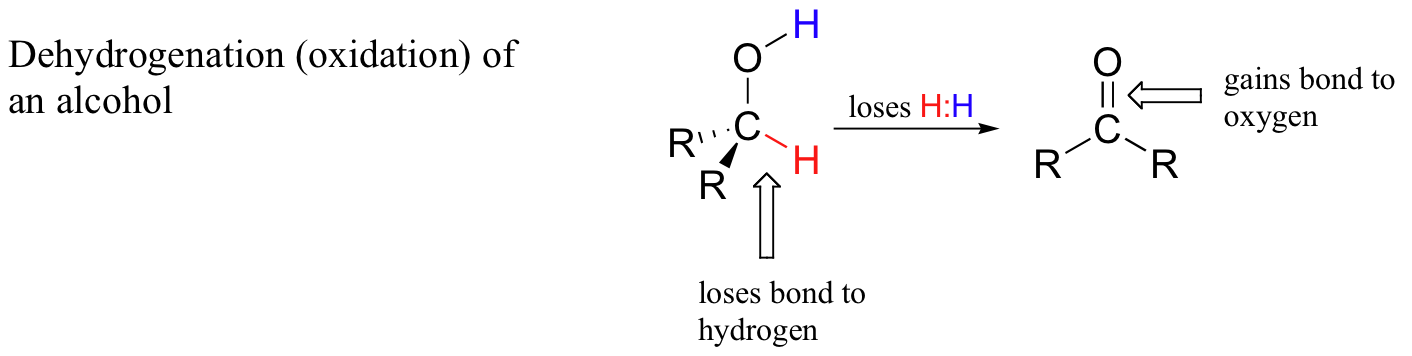
When a carbon atom loses a bond to hydrogen and gains a bond to a heteroatom (or to another carbon atom), it is considered to be an oxidative process because hydrogen, of all the elements, is the least electronegative. Thus, in the process of dehydrogenation the carbon atom undergoes an overall loss of electron density - and loss of electrons is oxidation.
Conversely, when a carbon atom in an organic compound gains a bond to hydrogen and loses a bond to a heteroatom (or to another carbon atom), we say that the compound has been hydrogenated, or reduced. The hydrogenation of a ketone to an alcohol, for example, is overall the reverse of the alcohol dehydrogenation shown above. Illustrated below is another common possibility, the hydrogenation (reduction) of an alkene to an alkane.
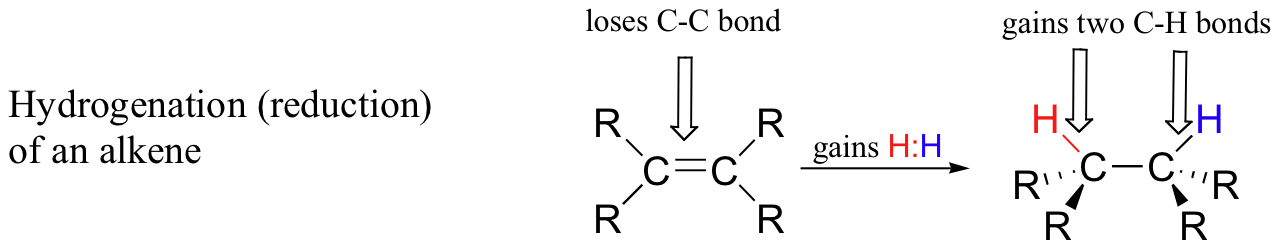
Hydrogenation results in higher electron density on a carbon atom(s), and thus we consider process to be one of reduction of the organic molecule.
Notice that neither hydrogenation nor dehydrogenation involves the gain or loss of an oxygen atom. Reactions which do involve gain or loss of one or more oxygen atoms are usually referred to as 'oxygenase' and 'reductase' reactions, and are the subject of section 16.10 and section 17.3.
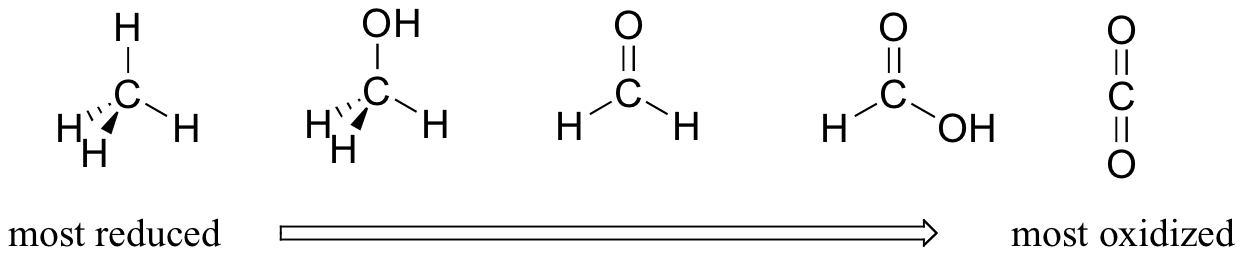
For the most part, when talking about redox reactions in organic chemistry we are dealing with a small set of very recognizable functional group transformations. It is therefore very worthwhile to become familiar with the idea of 'oxidation states' as applied to organic functional groups. By comparing the relative number of bonds to hydrogen atoms, we can order the familiar functional groups according to oxidation state. We'll take a series of single carbon compounds as an example. Methane, with four carbon-hydrogen bonds, is highly reduced. Next in the series is methanol (one less carbon-hydrogen bond, one more carbon-oxygen bond), followed by formaldehyde, formate, and finally carbon dioxide at the highly oxidized end of the group.
This pattern holds true for the relevant functional groups on organic molecules with two or more carbon atoms:
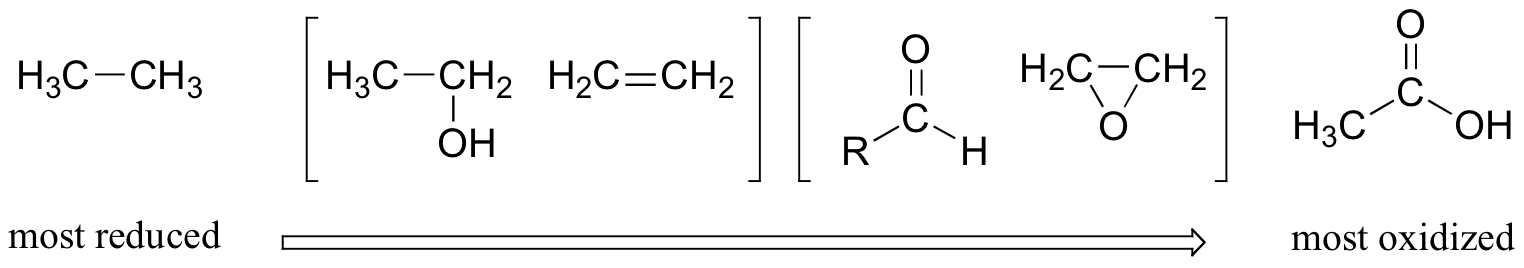
Alkanes are highly reduced, while alcohols - as well as alkenes, ethers, amines, sulfides, and phosphate esters - are one step up on the oxidation scale, followed by aldehydes/ketones/imines and epoxides, and finally by carboxylic acid derivatives (carbon dioxide, at the top of the oxidation list, is specific to the single carbon series).
Notice that in the series of two-carbon compounds above, ethanol and ethene are considered to be in the same oxidation state. You know already that alcohols and alkenes are interconverted by way of addition or elimination of water (section 14.1). When an alcohol is dehydrated to form an alkene, one of the two carbons loses a C-H bond and gains a C-C bond, and thus is oxidized. However, the other carbon loses a C-O bond and gains a C-C bond, and thus is considered to be reduced. Overall, therefore, there is no change to the oxidation state of the molecule.
You should learn to recognize when a reaction involves a change in oxidation state in an organic reactant . Looking at the following transformation, for example, you should be able to quickly recognize that it is an oxidation: an alcohol functional group is converted to a ketone, which is one step up on the oxidation ladder.
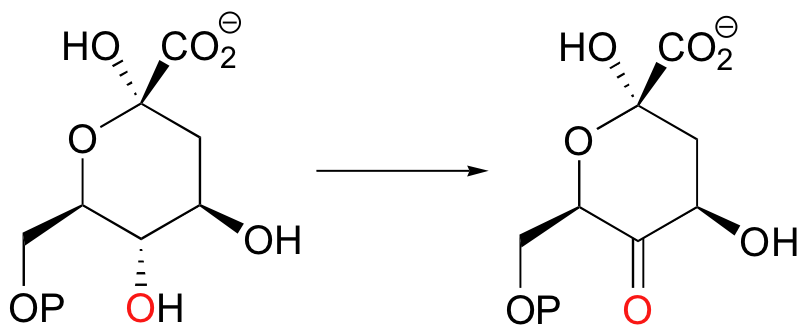
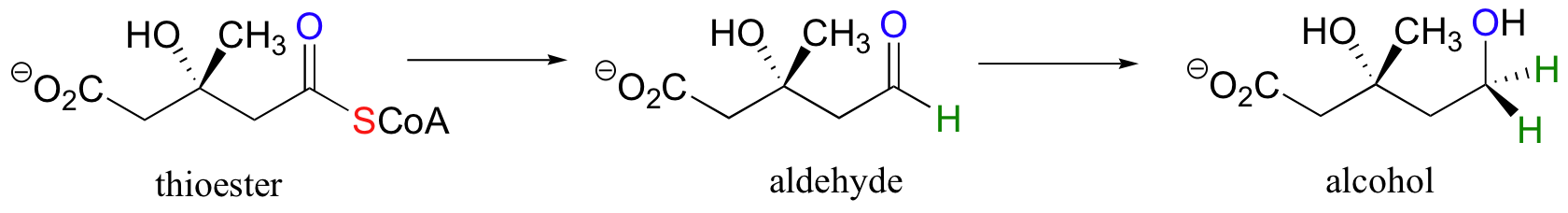
Likewise, this next reaction involves the transformation of a carboxylic acid derivative (a thioester) first to an aldehyde, then to an alcohol: this is a double reduction, as the substrate loses two bonds to heteroatoms and gains two bonds to hydrogens.
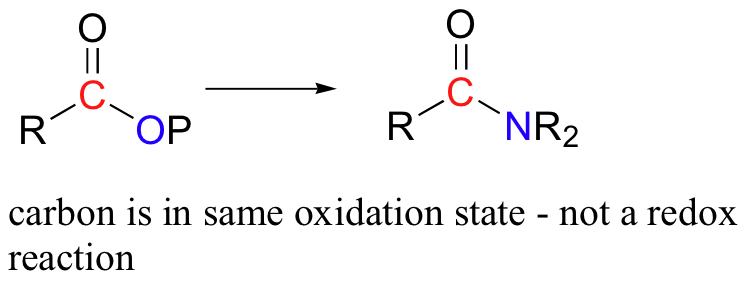
An acyl transfer reaction (for example the conversion of an acyl phosphate to an amide) is not considered to be a redox reaction - the oxidation state of the organic molecule is does not change as substrate is converted to product, because a bond to one heteroatom (oxygen) has simply been traded for a bond to another heteroatom (nitrogen).
It is important to be able to recognize when an organic molecule is being oxidized or reduced, because this information tells you to look for the participation of a corresponding redox agent that is being reduced or oxidized- remember, oxidation and reduction always occur in tandem! We will soon learn in detail about the most important biochemical and laboratory redox agents.
Exercise 16.1: is an aldol condensation a redox reaction? Explain.
Exercise 16.2: Is the reaction catalyzed by squalene epoxidase a redox reaction? How about squalene cyclase? Explain.
he combustion of carbon compounds, especially hydrocarbons, has been the most important source of heat energy for human civilizations throughout recorded history. The practical importance of this reaction cannot be denied, but the massive and uncontrolled chemical changes that take place in combustion make it difficult to deduce mechanistic paths. Using the combustion of propane as an example, we see from the following equation that every covalent bond in the reactants has been broken and an entirely new set of covalent bonds have formed in the products. No other common reaction involves such a profound and pervasive change, and the mechanism of combustion is so complex that chemists are just beginning to explore and understand some of its elementary features.
\[\ce{CH3 - CH2 - CH3} + 5 \ce{O2} \rightarrow 3 \ce{CO2} + 4\ce{H2O} + \text{heat}\]
Two points concerning this reaction are important:
- Since all the covalent bonds in the reactant molecules are broken, the quantity of heat evolved in this reaction is related to the strength of these bonds (and, of course, the strength of the bonds formed in the products). Precise heats of combustion measurements can provide useful information about the structure of molecules.
- The stoichiometry of the reactants is important. If insufficient oxygen is supplied some of the products will consist of the less oxidized carbon monoxide \(\ce{CO}\) gas.
\[\ce{CH3 - CH2 - CH3} + 4 \ce{O2} \rightarrow \ce{CO2} + 2 \ce{CO} + 4\ce{H2O} + \text{heat}\]
Heat of Combustion
From the previous discussion, we might expect isomers to have identical heats of combustion. However, a few simple measurements will disabuse this belief. Thus, the heat of combustion of pentane is –782 kcal/mole, but that of its 2,2-dimethylpropane (neopentane) isomer is –777 kcal/mole. Differences such as this reflect subtle structural variations, including the greater bond energy of 1º-C–H versus 2º-C–H bonds and steric crowding of neighboring groups. In small-ring cyclic compounds ring strain can be a major contributor to thermodynamic stability and chemical reactivity. The following table lists heat of combustion data for some simple cycloalkanes and compares these with the increase per CH2 unit for long chain alkanes.
| Cycloalkane (CH2)n | CH2 Units n | ΔH25º kcal/mole | ΔH25º per CH2 Unit | Ring Strain kcal/mole |
| Cyclopropane | n = 3 | 468.7 | 156.2 | 27.6 |
| Cyclobutane | n = 4 | 614.3 | 153.6 | 26.4 |
| Cyclopentane | n = 5 | 741.5 | 148.3 | 6.5 |
| Cyclohexane | n = 6 | 882.1 | 147.0 | 0.0 |
| Cycloheptane | n = 7 | 1035.4 | 147.9 | 6.3 |
| Cyclooctane | n = 8 | 1186.0 | 148.2 | 9.6 |
| Cyclononane | n = 9 | 1335.0 | 148.3 | 11.7 |
| Cyclodecane | n = 10 | 1481 | 148.1 | 11.0 |
| CH3(CH2)mCH3 | m = large | — | 147.0 | 0.0 |
The chief source of ring strain in smaller rings is angle strain and eclipsing strain. As noted elsewhere, cyclopropane and cyclobutane have large contributions of both strains, with angle strain being especially severe. Changes in chemical reactivity as a consequence of angle strain are dramatic in the case of cyclopropane, and are also evident for cyclobutane. Some examples are shown in the following diagram. The cyclopropane reactions are additions, many of which are initiated by electrophilic attack. The pyrolytic conversion of β-pinene to myrcene probably takes place by an initial rupture of the 1:6 bond, giving an allylic 3º-diradical, followed immediately by breaking of the 5:7 bond.
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry

